

偶氮次甲基亚胺与氮杂二烯前体的[4+3]环加成反应构建功能化四氮杂䓬衍生物
收稿日期: 2023-03-21
修回日期: 2023-05-15
网络出版日期: 2023-06-07
基金资助
贵州省科技厅基础研究计划(QKHJC-ZK[2023]-495); 重庆市自然科学基金(cstc2021jcyj-bsh0010)
Strategy to Construct Functionalized Tetrazepine Derivatives via [4+3] Annulation Reaction of Azomethine Imine with Azadiene Precursor
Received date: 2023-03-21
Revised date: 2023-05-15
Online published: 2023-06-07
Supported by
Guizhou Provincial Science and Technology Plan Program(QKHJC-ZK[2023]-495); Chongqing Natural Science Foundation(cstc2021jcyj-bsh0010)
张晓轲 , 郑相如 , 王朝永 . 偶氮次甲基亚胺与氮杂二烯前体的[4+3]环加成反应构建功能化四氮杂䓬衍生物[J]. 有机化学, 2023 , 43(9) : 3180 -3187 . DOI: 10.6023/cjoc202303031
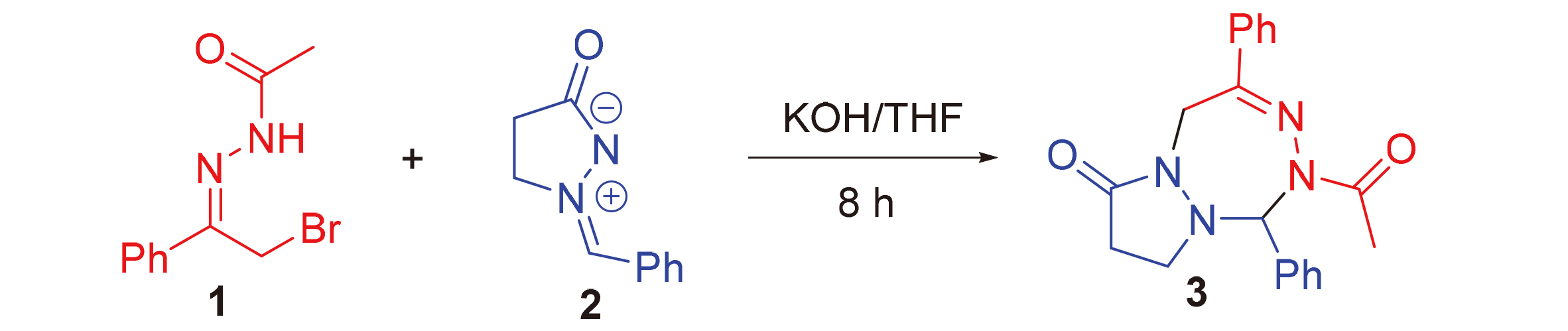
Tetrazepine derivative exhibits good biological activities. Only a limited method has been devoted to constructing these frameworks to date. Therefore, an efficient and novel way was established for the synthesis of functionalized tetrazepine scaffolds through the cycloaddition reaction of azomethine imine with 1,2-azadiene formed in situ by α-halogeno hydrazones in the presence of KOH. This transformation shows excellent functional group tolerance and substrate scope.
| [1] | For some selected examples: (a) Hu X.; Li, D.; Chu, C.; Li, X.; Wang, X. H.; Jia, Y.; Hua, H. M.; Xu, F. X. Int. J. Mol. Sci. 2018, 19, 3403. |
| [1] | (b) Xiao Y.; Gong Q.; Wang W. H.; Liu F.; Kong Q. H.; Pan F.; Zhang X. K.; Yu C. Y.; Hu S. S.; Fan F.; Li S. H.; Liu Y. Cancer Cell Int. 2020, 20, 371. |
| [1] | (c) Dong G. Q.; Liu Y.; Wu Y.; Tu J.; Chen S. Q.; Liu N.; Sheng C. Q. Chem. Commun. 2018, 54, 13535. |
| [1] | (d) Fu D. J.; Li J.; Yu B. Eur. J. Med. Chem. 2021, 214, 113254. |
| [1] | (e) Abdel-Halim M.; Sigler S.; Racheed N. A. S.; Hefnawy A.; Fathalla R. K.; Hammam M. A.; Maher A.; Maxuitenko Y. L.; Keeton A. B.; Hartmann R. W.; Engel M.; Piazza G. A.; Abadi A. H. J. Med. Chem. 2021, 64, 4462. |
| [2] | (a) Krueger B. W.; Fischer R.; Bertram H. J.; Bretschneider T.; Boehm S.; Krebs A.; Schenke T.; Santel H. J.; Lurssen K.; Schmidt R. R.; Erdelen C.; Wachendorff-Neumann U.; Stendel, W. EP0508126, 1992. |
| [2] | (b) Muehlebach M.; Cederbaum F.; Cornes D.; Friedmann A. A.; Glock J.; Hall G.; Indolese A. F.; Kloer D. P.; Goupil G. L.; Maetzke T.; Meier H.; Schneider R.; Stoller A.; Szczepanski H.; Wendeborna S.; Widmer H. Pest. Manage. Sci. 2011, 67, 1499. |
| [2] | (c) Kamata M.; Yamashita T.; Tokkyo Koho, J. K. JP 2009196966, 2009. |
| [2] | (d) Kamata M.; Yamashita T.; Kina A.; Tawada M.; Endo S.; Mizukami A.; Sasaki M.; Tani A.; Nakano Y.; Watanabe Y.; Furuyama N.; Funami M.; Amano N.; Fukatsu K. Bioorg. Med. Chem. Lett. 2012, 22, 4769. |
| [3] | (a) Kodato S. I.; Wada H.; Saito S.; Takeda M.; Nishibata Y.; Aoe K.; Date T.; Onoda Y.; Tamaki H. Chem. Pharm. Bull. 1987, 35, 80. |
| [3] | (b) Mashevskaya I. V.; Makhmudov R. R.; Kuslina L. V.; Mokrushin I. G.; Shurov S. N.; Maslivets A. N. Pharm. Chem. J. 2012, 45, 660. |
| [3] | (c) Ibrahim S. M.; Baraka M. M.; El-Sabbagh O. I.; Kothayer H. Med. Chem. Res. 2013, 22, 1488. |
| [4] | Almerico A. M.; Mingoia F.; Diana P.; Barraja P.; Lauria A.; Montalbano A.; Cirrincione G.; Dattolo G. J. Med. Chem. 2005, 48, 2859. |
| [5] | (a) Gupta C. M.; Bhaduri A. P.; Khanna N. M. J. Med. Chem. 1968, 11, 392. |
| [5] | (b) Ghorab M. M.; Heiba H. I.; El-gawish M. A. Phosphorus, Sulfur Silicon Relat. Elem. 1995, 106, 85. |
| [5] | (c) Hamama W. S.; El-Bana G. G.; Shaaban S.; Zoorob H. H. J. Heterocycl. Chem. 2018, 55, 971. |
| [6] | (a) Maggio B.; Raffa D.; Raimondi M. V.; Cascioferro S.; Plescia F.; Tolomeo M.; Barbusca E.; Cannizzo G.; Mancuso S.; Daidone G. Eur. J. Med. Chem. 2008, 43, 2386. |
| [6] | (b) Maggio B.; Raffa D.; Raimondi M. V. ARKIVOC 2006, xv, 120. |
| [6] | (c) Vahedi H.; Rajabzadeh G.; Farvandi F. Chin. Chem. Lett. 2010, 21, 1419. |
| [6] | (d) Darehkordi A.; Khorasani F. N.; Mohammadi M.; Kazemi E. Monatsh. Chem. 2020, 151, 1835. |
| [7] | For selected examples, see: (a) Zhou M. B.; Song, R. J.; Wang, C. Y.; Li, J. H. Angew. Chem., Int. Ed. 2013, 52, 10805. |
| [7] | (b) Feng J. J.; Lin T. Y.; Wu H. H.; Zhang J. L. J. Am. Chem. Soc. 2015, 137, 3787. |
| [7] | (c) Liu L. L.; Chiu P. Chem. Commun. 2011, 47, 3416. |
| [7] | (d) Gao H. Y.; Wu X. X.; Zhang J. L. Chem. Commun. 2010, 46, 8764. |
| [8] | Kumari Y. B. Heterocycl. Commun. 2007, 13, 177. |
| [9] | Li Z. F.; Li S. K.; Kan T. J.; Wang X. Y.; Xin X.; Hou Y. L.; Gong P. Adv. Synth. Catal. 2020, 362, 2626. |
| [10] | (a) Huisgen R.; Fleischmann R.; Eckell A. Tetrahedron Lett. 1960, 1, 1. |
| [10] | (b) Breugst M.; Reissig H. U. Angew. Chem., Int. Ed. 2020, 59, 12293. |
| [10] | (c) Yue G. Z.; Liu B. Chin. J. Org. Chem. 2020, 40, 3132. (in Chinese) |
| [10] | (乐贵洲, 刘波, 有机化学, 2020, 40, 3132.) |
| [11] | (a) Bakthadoss M.; Agarwal V. ChemistrySelect 2018, 3, 6960. |
| [11] | (b) Choi A.; Castle J.; Saruengkhanphasit R.; Coldham I. Synthesis 2020, 1273. |
| [11] | (c) Sandmeier T.; Sievertsen N.; Carreira E. M. Helv. Chim. Acta 2020, 103, e2000058. |
| [12] | (a) Li Z.; Yu H.; Liu Y.; Zhou L.; Sun Z.; Guo H. Adv. Synth. Catal. 2016, 358, 1880. |
| [12] | (b) Zhou L.; Yuan C.; Zhang C.; Zhang L.; Gao Z.; Wang C.; Liu H.; Wu Y.; Guo H. Adv. Synth. Catal. 2017, 359, 2316. |
| [12] | (c) Zhu C. Z.; Feng J. J.; Zhang J. Chem. Commun. 2017, 53, 4688. |
| [12] | (d) Yu L.; Zhong Y.; Yu J.; Gan L.; Cai Z.; Wang R.; Jiang X. Chem. Commun. 2018, 54, 2353. |
| [12] | (e) Cheng X.; Cao X.; Xuan J.; Xiao W. J. Org. Lett. 2018, 20, 52. |
| [13] | (a) Wei L.; Wang Z. F.; Yao L.; Qiu G. F.; Tao H. Y.; Li H.; Wang C. J. Adv. Synth. Catal. 2016, 358, 3955. |
| [13] | (b) Wang Y. F.; Zhu L. P.; Wang M. R.; Xiong J.; Chen N. N.; Feng X.; Xu Z. Q.; Jiang X. X. Org. Lett. 2018, 20, 6506. |
| [14] | (a) Li Z.; Feng Y. L.; Hou Z. F.; Zhang L.; Yang W. J.; Wu Y.; Xiao Y. M.; Guo H. C. RSC Adv. 2015, 5, 34481. |
| [14] | (b) Mei G. J.; Zhu Z. Q.; Zhao J. J.; Bian C. Y.; Chen J.; Chen R. W.; Shi F. Chem. Commun. 2017, 53, 2768. |
| [14] | (c) Li C.; Wang C. S.; Li T. Z.; Mei G. J.; Shi F. Org. Lett. 2019, 21, 598. |
| [14] | (d) Yuan C. H.; Zhou L. J.; Xia M. R.; Sun Z. H.; Wang D. Q.; Guo H. C. Org. Lett. 2016, 18, 5644. |
| [14] | (e) Na R.; Jing C.; Xu Q.; Jiang H.; Wu X.; Shi J. J. Am. Chem. Soc. 2011, 133, 13341. |
| [14] | (f) Wang M.; Huang Z. J.; Xu J. F.; Chi Y. R. J. Am. Chem. Soc. 2014, 136, 1214. |
| [14] | (g) Na R.; Jing C. F.; Xu Q. H.; Jiang H.; Wu X.; Shi J. Y.; Zhong J. C.; Wang M.; Benitez D.; Tkatchouk E.; Goddard W. A.; Guo H. C.; Kwon O. J. Am. Chem. Soc. 2011, 133, 13337. |
| [14] | (h) Hu X. Q.; Chen J. R.; Gao S.; Feng B.; Lu L. Q.; Xiao W. J. Chem. Commun. 2013, 49, 7905. |
| [14] | (i) Yang W. J.; Yuan C. H.; Liu Y.; Mao B. M.; Sun Z. H.; Guo H. C. J. Org. Chem. 2016, 81, 7597. |
| [15] | (a) Jin Q.; Zhang J.; Jiang C.; Zhang D.; Gao M.; Hu S. J. Org. Chem. 2018, 83, 8410. |
| [15] | (b) Hu X. Q.; Chen J. R.; Gao S.; Feng B.; Lu L. Q.; Xiao W. J. Chem. Commun. 2013, 49, 7906. |
| [15] | (c) Zhi Y.; Zhao K.; Shu T.; Enders D. Synthesis 2016, 48, 240. |
| [15] | (d) Chen L.; Yang G. M.; Wang J.; Jia Q. F.; Wei J.; Du Z. Y. RSC Adv. 2015, 5, 76697. |
| [16] | For selected examples, see: (a) Zhang X. K.; Pan, Y.; Wang, H. B.; Liang, C.; Ma, X. F.; Jiao, W.; Shao, H. W. Adv. Synth. Catal. 2021, 363, 459. |
| [16] | (b) Chen J. R.; Dong W. R.; Candy M.; Pan F. F.; Jo?rres M.; Bolm C. J. Am. Chem. Soc. 2012, 134, 6924. |
| [16] | (c) Huang R.; Chang X.; Li J.; Wang C. J. J. Am. Chem. Soc. 2016, 138, 3998. |
| [16] | (d) Quan B. X.; Zhuo J. R.; Zhao J. Q.; Zhang M. L.; Zhou M. Q.; Zhang X. M.; Yuan W. C. Org. Biomol. Chem. 2020, 18, 1886. |
| [16] | (f) Chen B.; Chu W. D.; Liu Q. Z. RSC Adv. 2019, 9, 1487. |
| [16] | (g) Huang R.; Chang X.; Li J.; Wang C. J. J. Am. Chem. Soc. 2016, 138, 3998. |
| [16] | (h) Yin W. H.; Fang L.; Wang Z. Y.; Gao F.; Li Z. F.; Wang Z. Y. Org. Lett. 2020, 21, 7361. |
| [16] | (i) Cao W. B.; Xu X. P.; Ji S. J. Org. Biomol. Chem. 2017, 15, 1651. |
| [16] | (j) Zhong X. G.; Lv J.; Luo S. Org. Lett. 2015, 17, 1561. |
| [16] | (k) Deng Y. M.; Pei C.; Arman H.; Dong K. Y.; Xu X. F.; Doyle M. P. Org. Lett. 2016, 18, 5884. |
| [17] | Zhao H. W.; Pang H. L.; Tian T.; Li B.; Chen X. Q.; Song X. Q.; Meng W.; Yang Z.; Liu Y. Y.; Zhao Y. D. Adv. Synth. Catal. 2016, 358, 1826. |
| [18] | Cheng B.; Li Y. T.; Wang T. M.; Zhang X. P.; Li H.; Li Y.; Zhai H. B. Chem. Commun. 2019, 55, 14606. |
| [19] | Zhang X. K.; Wang H. B.; Li Z. W.; Shu Y.; Gan S. Zhang X. F.; Shao H. W.; Wang C. Y. ACS Omega 2022, 7, 40963. |
/
| 〈 |
|
〉 |