

铜催化格氏试剂的不对称烯丙基烷基化连续流反应
收稿日期: 2023-03-20
修回日期: 2023-04-24
网络出版日期: 2023-06-14
基金资助
深圳市基础研究专项(JCYJ20190806142203709); 深圳市基础研究专项(JSGG20191129114029286); 深圳市基础研究专项(JSGG20201103153807021); 深圳市基础研究专项(GXWD2022081117373600); 广东省基础与应用基础研究基金(2021A1515110366); 中国博士后科学基金(2022M720951); 深圳市先进功能碳基材料研究与综合应用重点实验室资助项目
Copper-Catalyzed Asymmetric Allyl Alkylation Using Grignard Reagents under Continuous Flow
Received date: 2023-03-20
Revised date: 2023-04-24
Online published: 2023-06-14
Supported by
Shenzhen Basic Research Foundation(JCYJ20190806142203709); Shenzhen Basic Research Foundation(JSGG20191129114029286); Shenzhen Basic Research Foundation(JSGG20201103153807021); Shenzhen Basic Research Foundation(GXWD2022081117373600); Guangdong Basic and Applied Basic Research Foundation(2021A1515110366); China Postdoctoral Science Foundation(2022M720951); Shenzhen Key Laboratory of Advanced Functional Carbon Materials Research and Comprehensive Application
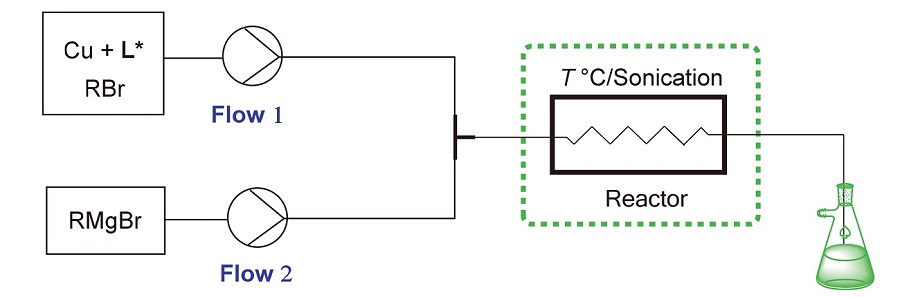
基于连续流微通道反应器, 在温和条件下首次实现了铜催化格氏试剂的不对称烯丙基烷基化反应. 在–20~ –10 ℃条件下, 用双流路不锈钢折线型连续流微通道反应器, 以极短的保留时间0.6 s, 实现了铜催化的不同格氏试剂的不对称烯丙基烷基化反应, 获得的产物收率为82%~99%, ee值中等至良好. 此外, 在连续流反应条件下连续进料34 min, 放大(S)-3-甲基-1-环己烯的合成规模, 获得0.968 g的(S)-3-甲基-1-环己烯, 收率为98.7%, ee值为96.6%, 证明了在连续流条件下铜催化剂可以稳定地催化格氏试剂发生不对称烯丙基烷基化反应.
宋晓 , 卿晶 , 黎君 , 贾雪雷 , 吴福松 , 黄均荣 , 金剑 , 游恒志 . 铜催化格氏试剂的不对称烯丙基烷基化连续流反应[J]. 有机化学, 2023 , 43(9) : 3174 -3179 . DOI: 10.6023/cjoc202303027
Continuous flow reactors were used to explore the copper-catalyzed asymmetric allyl alkylation (AAA) reactions of Grignard reagents under milder conditions for the first time. After continuous optimization, a 2-channel stainless steel broken-line continuous flow reactor was used to achieve a copper-catalyzed AAA reaction of various Grignard reagents at –20~–10 ℃ under very short reaction retention time of 0.6 s furnishing the corresponding products in 82%~99% yields with moderate to good enantioselectivity. Furthermore, the copper-catalyzed AAA reaction of 3-bromocyclohexene with methylmagnesium bromide was scaled up under a continuous flow process with continuous feeding for 34 min affording 0.968 g of (S)-3-methylcyclohex-1-ene with 98.7% yield and 96.6% ee.

| [1] | Trost B. M.; Crawley M. L. Chem. Rev. 2003, 103, 2921. |
| [2] | Cui P. L.; Liu H. Y.; Zhang D. N.; Wang C. Chin. J. Org. Chem. 2012, 32, 1401. (in Chinese) |
| [2] | (崔朋雷, 刘海燕, 张冬暖, 王春, 有机化学, 2012, 32, 1401.) |
| [3] | Li J.; Song X.; Wu F.; You H.; Chen F.-E. Eur. J. Org. Chem. 2022, e202200860. |
| [4] | Klaveren M.; Persson E. S. M.; del Villar A.; Grove D. M.; B?ckvall J.-E.; van Koten G. Tetrahedron Lett. 1995, 36, 3059. |
| [5] | Meuzelaar G. J.; Karlstr?m A. S. E.; van Klaveren M.; Persson E. S. M.; del Villar A.; van Koten G.; B?ckvall J.-E. Tetrahedron 2000, 56, 2895. |
| [6] | Karlstr?m A. S. E.; Huerta F. F.; Meuzelaar G. J.; B?ckvall J.-E. Synlett 2001, 2001(Special Issue),0923. |
| [7] | Langlois J.-B.; Alexakis A. Adv. Synth. Catal. 2010, 352, 447. |
| [8] | Plutschack M. B.; Pieber B.; Gilmore K.; Seeberger P. H. Chem. Rev. 2017, 117, 11796. |
| [9] | Adamo A.; Beingessner R. L.; Behnam M.; Chen J.; Jamison T. F.; Jensen K. F.; Monbaliu J. C. M.; Myerson A. S.; Revalor E. M.; Snead D. R. Science 2016, 352, 61. |
| [10] | Lin H.; Dai C.; Jamison T. F.; Jensen K. F. Angew. Chem., Int. Ed. 2017, 56, 8870. |
| [11] | Yao H.; Wan L.; Zhao X.; Guo Y.; Zhou J.; Bo X.; Mao Y.; Xin Z. Org. Process Res. Dev. 2021, 25, 2060. |
| [12] | Liao J.; Zhang S.; Wang Z.; Song X.; Zhang D.; Kumar R.; Jin J.; Ren P.; You H.; Chen F. Green Synth. Catal. 2020, 1, 121. |
| [13] | Coley C. W.; Thomas D. A.; Lummiss J.; Jaworski J. N.; Jensen K. F. Science 2019, 365, eaax1566. |
| [14] | Zisu B.; Bhaskaracharya R.; Kentish S.; Ashokkumar M. Ultrason. Sonochem. 2010, 17, 1075. |
| [15] | You H.; Rideau E.; Sidera M.; Fletcher S. Nature 2015, 517, 351. |
/
| 〈 |
|
〉 |