

钴催化芳基烯烃氧烷基化反应: 快速获得α-烷基取代苯乙酮衍生物
收稿日期: 2023-04-07
修回日期: 2023-06-15
网络出版日期: 2023-07-06
基金资助
茂名绿色化工研究院“扬帆计划”(MMGCIRI-2022YFJH-Y-037); 茂名市科技计划(2022031); 广东省普通高校重点领域专项(2020-ZDZX2054)
Cobalt-Catalyzed Oxyalkylation Reaction of Styrenes: Rapid Access to α-Alkyl Substituted Acetophenone Derivatives
Received date: 2023-04-07
Revised date: 2023-06-15
Online published: 2023-07-06
Supported by
Sailing Plan of Maoming Green Chemical Industry Research Institute(MMGCIRI-2022YFJH-Y-037); Science and Technology Planning Project of Maoming City(2022031); Special Projects in Key Fields of Ordinary Universities of Guangdong Province(2020-ZDZX2054)
周鹏 , 朱伟明 , 张建涛 , 肖朵朵 , 郭祥峰 , 刘卫兵 . 钴催化芳基烯烃氧烷基化反应: 快速获得α-烷基取代苯乙酮衍生物[J]. 有机化学, 2023 , 43(11) : 3939 -3944 . DOI: 10.6023/cjoc202304010
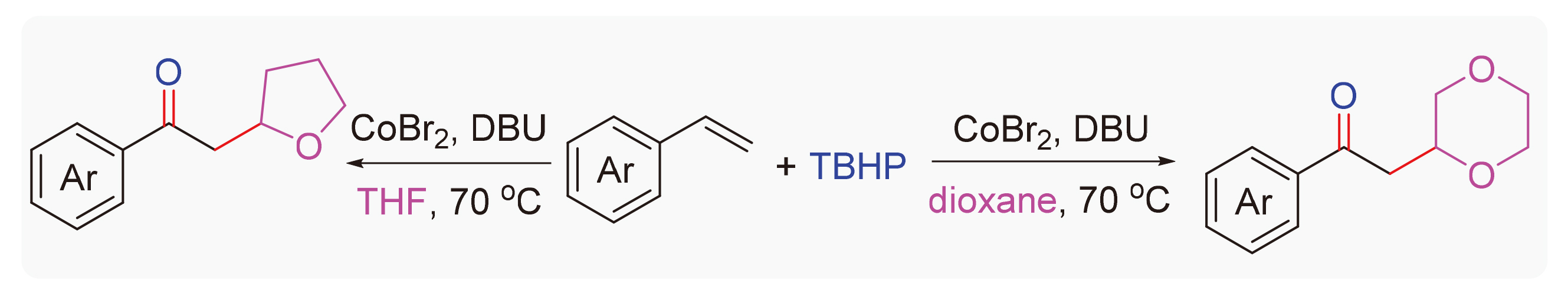
Alkyl aryl ketones are a class of valuable compounds in chemistry and biology, which are widely used in the pharmaceutical, spice, dye, pesticide, functional materials, and organic synthesis industries. A cobalt-catalyzed oxyalkylation reaction of aryl olefin with tert-butyl hydroperoxide (TBHP) and cyclic ethers was developed. Various substrates with electron-withdrawing or electron-donating groups were tolerated in this protocol to provide the α-alkyl substituted aceto- phenones in moderate yields. The present protocol features broad substrate scope, and flexibility, allowing rapid assembly of a series of value-added acetophenone derivatives under mild conditions with good reaction efficacy.
| [1] | Liang, Q.; Qian, M.; Razzak, M.; De Brabander, J. K. Chem.- Asian J. 2011, 6, 1958. |
| [2] | Zhang, J.-X.; Wang, Y.-J.; Zhang, W.; Wang, N.-X.; Bai, C.-B.; Xing, Y.-L.; Li, Y.-H.; Wen, J.-L. Sci. Rep. 2014, 4, 7446. |
| [3] | Hua, Z.; Tang, Y.; Zhang, S.; Li, X.; Dua, X.; Xu, X. Synlett 2015, 26, 2557. |
| [4] | (a) Ji, J.; Liu, P.; Sun, P. Chem. Commun. 2015, 51, 7546. |
| [4] | (b) Lan, X.-W.; Wang, N.-X.; Bai, C.-B.; Lan, C.-L.; Zhang, T.; Chen, S.-L.; Xing, Y. Org. Lett. 2016, 18, 5986. |
| [4] | (c) Zhang, S.-L.; Wang, X.-J.; Yu, Z.-L. Org. Lett. 2017, 19, 3139. |
| [4] | (d) Yadava, A. K.; Singh, K. N. Chem. Commun. 2018, 54, 1976. |
| [4] | (e) Wang, J.-Y.; Ma, L.; Li, Y.; Wang, X.-S. Chin. J. Org. Chem. 2019, 39, 232. (in Chinese) |
| [4] | (王建勇, 马岚, 李彦, 王细胜, 有机化学, 2019, 39, 232.) |
| [4] | (f) Chen, R.; Wang, Y.; Wang, Z.-Y.; Ma, X.; Xu, C.; Wang, K.-K.; Sun, A. Tetrahedron Lett. 2022, 98, 153807. |
| [5] | Ji, P.-Y.; Liu, Y.-F.; Xu, J.-W.; Luo, W.-P.; Liu, Q.; Guo, C.-C. J. Org. Chem. 2017, 82, 2965. |
| [6] | Zhang, H.-Y.; Ge, C.; Zhao, J.; Zhang, Y. Org. Lett. 2017, 19, 5260. |
| [7] | Qu, Z.; Tian, T.; Tan, Y.; Ji, X.; Deng, G.-J.; Huang, H. Green Chem. 2022, 24, 7403. |
| [8] | Gao, Y.; Qin, W.; Tian, M.-Q. Zhao, X.; Hu, X.-H. Adv. Synth. Catal. 2022, 364, 2241. |
| [9] | Zhang, X.; Li, Y. Org. Lett. 2022, 24, 8057. |
| [10] | Cheng, K.; Huang, L.; Zhang, Y. Org. Lett. 2009, 11, 2908. |
| [11] | Sun, H.; Zhang, Y.; Guo, F.; Zha, Z.; Wang, Z. J. Org. Chem. 2012, 77, 3563. |
| [12] | Li, J.; Li, J.; Yuan, S.; Zhang, Q.; Li, D. Asian J. Org. Chem, 2019, 8, 1842. |
| [13] | Jiao, Y.; Chiou, M.-F.; Li, Y.; Bao, H. ACS Catal. 2019, 9, 5191. |
| [14] | Kibriya, G.; Ghosh, D.; Hajra, A. Sci. China: Chem. 2020, 63, 42. |
| [15] | Chen, R.; Wang, K.-K.; Wang, Z.-Y.; Ma, X.; Wang, D.; Zhang, A.; Liu, L. ChemistrySelect 2020, 5, 2078. |
| [16] | (a) Zhang, H.; Xiao, Q.; Qi, X.-K.; Gao, X.-W.; Tong, Q.-X.; Zhong, J.-J. Chem. Commun. 2020, 56, 12530. |
| [16] | (b) Xiao, Q.; Zhang, H.; Li, J.-H.; Jian, J.-X.; Tong, Q.-X.; Zhong, J.-J. Org. Lett. 2021, 23, 3604. |
| [16] | (c) Xiao, Y.; Zhu, C.-M.; Liang, R.-B.; Huang, Y.-L.; Hai, C.-H.; Chen, J.-R.; Li, M.; Zhong, J.-J.; Huang, X.-C. Chem. Commun. 2023, 59, 2239. |
| [17] | Zhang, J.; Xiao, D.; Tan, H.; Liu, W. J. Org. Chem. 2020, 85, 3929. |
| [18] | Chen, C.; Tan, H.; Liu, B.; Yue, C.; Liu, W. Org. Chem. Front. 2018, 5, 3143. |
| [19] | Chen, C.; Li, Y.; Pan, Y.; Duan, L.; Liu, W. Org. Chem. Front. 2019, 6, 2032. |
| [20] | Zhang, J.; Zhou, P.; Yin, A.; Zhang, S.; Liu, W. J. Org. Chem. 2021, 86, 8980. |
| [21] | Xiao, D.; Liu, H.; Zhou, P.; Zhang, J.; Liu, W. Chin. J. Org. Chem. 2022, 42, 1438. (in Chinese) |
| [21] | (肖朵朵, 刘海灵, 周鹏, 张建涛, 刘卫兵, 有机化学, 2022, 42, 1438.) |
/
| 〈 |
|
〉 |