

N,O-配体钴化合物的合成及其环氧丙烷羰化酯化的催化性能
收稿日期: 2023-04-12
修回日期: 2023-06-21
网络出版日期: 2023-07-06
基金资助
国家自然科学基金面上(21972112); 福建省高校产学研(2021H6002)
Synthesis and Propylene Oxide Carbonylation Hydroesterification Catalytic Property of N,O-Ligand Coordination Cobalt Compounds
Received date: 2023-04-12
Revised date: 2023-06-21
Online published: 2023-07-06
Supported by
National Natural Science Foundation of China(21972112); Productive and Researching Foundation of Fujian Province(2021H6002)
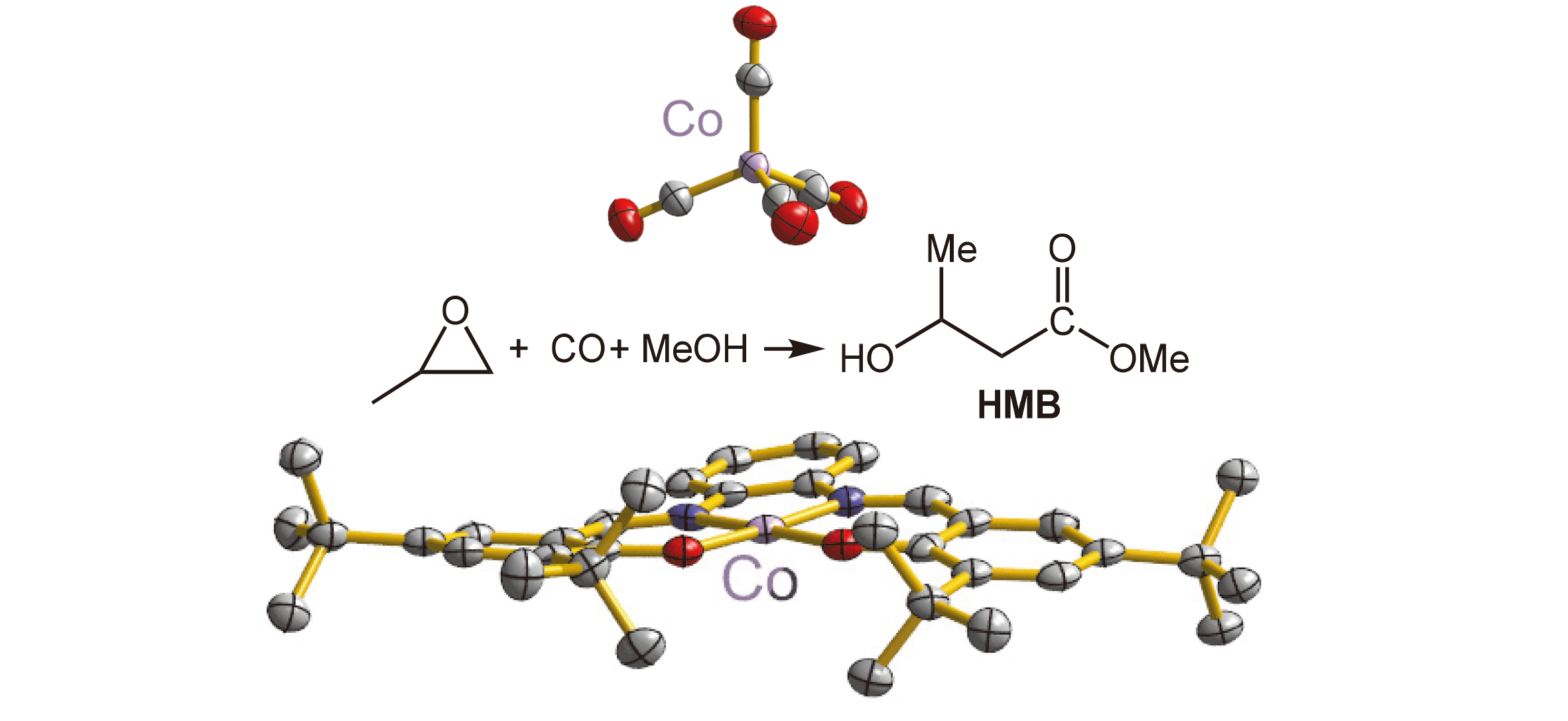
合成了9种N,O-配体化合物L1~L9. 化合物L1~L4分别与0.5 equiv. Co2(CO)8发生氧化还原配位反应生成中性单核钴化合物1~4; L5~L7分别与1 equiv. Co2(CO)8发生歧化和氧化还原配位反应; L8与5/6 equiv. Co2(CO)8以及1.2 equiv. MeOH和0.4 equiv. H2O发生歧化和氧化还原配位反应; L9与0.5 equiv. Co2(CO)8发生歧化配位反应生成同钴核离子对化合物5~9. 这些化合物中的阴离子均为[Co(CO)4]–. 相应地, 化合物8中的阳离子是三核钴簇, 其它化合物中的阳离子都是单核钴. 化合物1~9通过FT-IR谱学表征和元素分析数据确定, 其中1和8进一步经过X射线单晶结构确认.考察了化合物1~9催化环氧丙烷(PO)羰化酯化的性能, 获得42.6%~99.0%的PO转化率和34.1%~88.6%的β-羟基丁酸甲酯(HMB)总产率. 研究了8和9随时间变化的催化反应, 推测了同钴核离子对协同作用的催化反应机理.
李泽辉 , 邹昊宇 , 李林才 , 赵怡玲 , 朱红平 . N,O-配体钴化合物的合成及其环氧丙烷羰化酯化的催化性能[J]. 有机化学, 2023 , 43(11) : 3907 -3915 . DOI: 10.6023/cjoc202304016
Nine N,O-ligands L1~L9 have been synthesized. L1~L4 each reacted with half equivalent Co2(CO)8 by redox coordination to produce neutral mononuclear cobalt compounds 1~4. Reactions of L5~L9 each with equivalent Co2(CO)8 occurred by disproportionation and redox coordination to afford 5~7. When the reaction of L8, 5/6 equiv. of Co2(CO)8, 1.2 equiv. of MeOH, and 0.4 equiv. of H2O took place via disproportionation and redox coordination to give compound 8, L9 and 0.5 equiv. of Co2(CO)8 happened through disproportionation coordination to form compound 9. Compounds 5~9 are all the ionic species with the same anion [Co(CO)4]–, but coupled by mononuclear cobalt cation for 5~7 and 9 whereas trinuclear cation for 8. Compounds 1~9 have been characterized by FT-IR spectroscopies and elemental analysis, of which 1 and 8 were further determined by X-ray crystallography. The catalytic property of propylene oxide (PO) carbonylation hydroesterification using 1~9 was investigated, achieving PO conversions of 42.6%~99.0% and β-hydroxy methylbutyrate (HMB) yields of 34.1%~88.6%. The time-traced reactions were carried out for both catalysts 8 and 9, and the catalytic reaction mechanism based on cooperative interplay by the homonuclear cobalt ion pair was discussed as well.

| [1] | Zhang, J.; Qian, C.; Li, S.; Lu, X.; Zhao, Y.; Guan, J. S.; Chen, J. C.; Wu, Q.; Chen, G. Q. Biomaterials 2013, 34, 7552. |
| [2] | Eisenmann, J.; Yamartino, R.; Howard, J. J. J. Org. Chem. 1961, 26, 2102. |
| [3] | Sun, Y.; Ma, P.-S. Tianjin Chem. Ind. 2006, 20, 40. (in Chinese) |
| [3] | (孙义, 马沛生, 天津化工, 2006, 20, 40.) |
| [4] | Zhu, J.-H.; Yu, Z.-Z.; Zhang, F. CN 103012149, 2013. |
| [5] | Ni, H.-L.; Yao, S.-J. Chem. React. Eng. Technol. 2007, 173. (in Chinese) |
| [5] | (倪宏亮, 姚善泾, 化学反应工程与工艺, 2007, 173.) |
| [6] | Li, X.-Q.; Tang, Y.-C.; Xu, X.-S. CN 105001070, 2015. |
| [7] | Zhao, J.; Wu, P.; Lai, E.; Li, J.; Jiang, W.; Wang, B.; Zhu, H. Chem.-Asian J. 2021, 16, 3453. |
| [8] | Getzler, Y. D. Y. L.; Mahadevan, V.; Lobkovsky, E. B.; Coates, G. W. J. Am. Chem. Soc. 2002, 124, 1174. |
| [9] | Veronese, L.; Brivio, M.; Biagini, P.; Po, R.; Tritto, I.; Losio, S.; Boggioni, L. Organometallics 2020, 39, 2653. |
| [10] | Chen, F.-X.; Zhou, H.; Liu, X.; Qin, B.; Feng, X.; Zhang, G.; Jiang, Y. Chem.-Eur. J. 2004, 10, 4790. |
| [11] | Hosseini-Monfared, H.; Soleymani-Babadi, S.; Sadighian, S.; Pazio, A.; Wozniak, K.; Siczek, M.; Mayer, P. Transition Met. Chem. 2015, 40, 255. |
| [12] | Kramer, J. W.; Lobkovsky, E. B.; Coates, G. W. Org. Lett. 2006, 8, 3709. |
| [13] | Tuba, R.; Mika, L. T.; Bodor, A.; Pusztai, Z.; Tóth, I.; Horváth, I. T. Organometallics 2003, 22, 1582. |
| [14] | Wender, I.; Sternberg, H. W.; Orchin, M. J. Am. Chem. Soc. 1953, 75, 3041. |
| [15] | Liu, R.; Zhong, X.; Liu, Z.; Liang, S.; Zhu, H. Chin. J. Org. Chem. 2017, 37, 2315. (in Chinese) |
| [15] | (刘睿, 钟向宏, 刘振宇, 梁胜彪, 朱红平, 有机化学, 2017, 37, 2315.) |
| [16] | Lamb, J. R.; Mulzer, M.; LaPointe, A. M.; Coates, G. W. J. Am. Chem. Soc. 2015, 137, 15049. |
| [17] | Yang, J.-C.; Yang, J.; L, W.-B.; L, X.-B.; L, Y. Angew. Chem., Int. Ed. 2022, 61, e202116208. |
| [18] | Rajendiran, R.; Gunasekar, G. H.; Yoon, S. New J. Chem. 2018, 42, 12256. |
| [19] | Wender, I.; Sternberg, H. W.; Orchin, M. J. Am. Chem. Soc. 1952, 74, 1216. |
/
| 〈 |
|
〉 |