

碘促进N,N-二甲基乙酰胺(DMA)与胺的转酰胺反应
收稿日期: 2023-04-18
修回日期: 2023-06-06
网络出版日期: 2023-07-06
基金资助
国家自然科学基金(31701679); 陕西省科学技术协会青年人才托举计划(20220609); 陕西省科技资源开放共享平台(2021PT-004); 延安市科技计划(2022SLSFGG-005); 延安市科学技术协会青年人才托举计划(8); 延安大学大学生创新创业训练计划(D2021032); 延安大学产学研合作培育(CXY202102); 陕西省教育厅青年创新团队(23JP193); 陕西高校青年创新团队资助项目
Iodine-Promoted Transamidation of N,N-Dimethylacetamide (DMA) with Amines
Received date: 2023-04-18
Revised date: 2023-06-06
Online published: 2023-07-06
Supported by
National Natural Science Foundation of China(31701679); Young Talent Fund of Association for Science and Technology in Shaanxi(20220609); Open Sharing Platform for Scientific and Technological Resources of Shaanxi Province(2021PT-004); Yan'an Science and Technology Planning Project(2022SLSFGG-005); the Young Talent Fund of Association for Science and Technology in Yanan(8); Yan'an University Training Program of Innovation and Entrepreneurship for Undergraduates(D2021032); Yan'an University Industry-University-Research Co-operative Cultivation Project(CXY202102); Youth Innovation Team Project of Shaanxi Provincial Education Department(23JP193); Youth Innovation Team of Shaanxi Universities
马豪杰 , 周风院 , 苏凡文 , 韩波 , 李然 , 张玉琦 , 王记江 . 碘促进N,N-二甲基乙酰胺(DMA)与胺的转酰胺反应[J]. 有机化学, 2023 , 43(11) : 3960 -3965 . DOI: 10.6023/cjoc202304022
A novel and convenient transamidation of N,N-dimethylacetamide (DMA) with amines for the synthesis of acetamides with moderate to excellent yields is reported. Low-cost I2 is used as efficient promoter for this transformation. Furthermore, the reaction has simple conditions, good functional group tolerance, substrates without prefunctionalization, and practicality, which can be widely used in the synthesis of acetamide drugs and biologically active molecules.
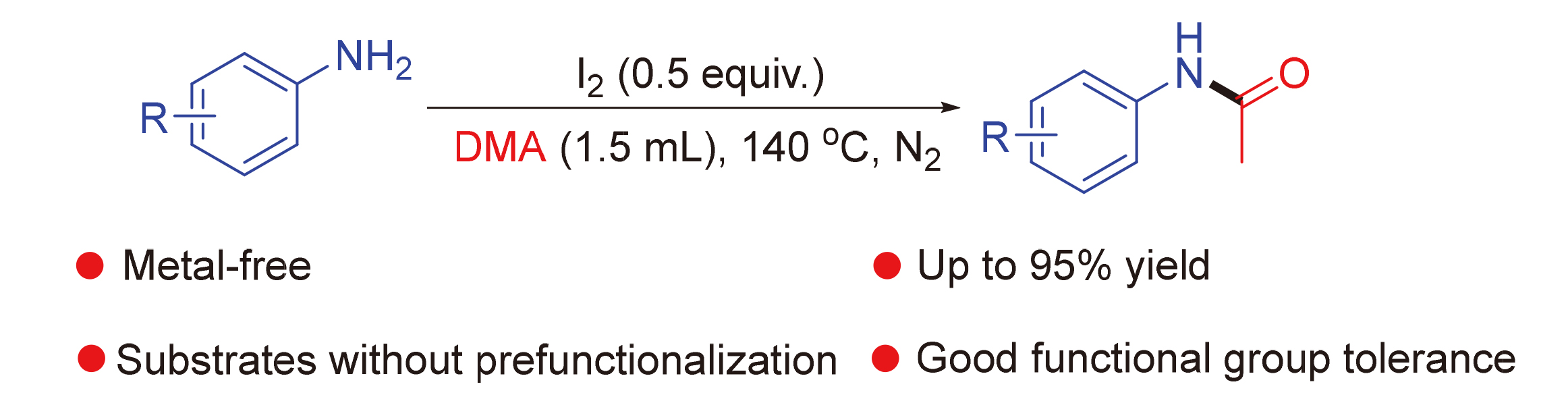
Key words: iodine; N-acylation; transamidation; N; N-dimethylacetamide; acetamide
| [1] | (a) Nagaraaj, P.; Vijayakumar, V. Org. Chem. Front. 2019, 6, 2570. |
| [1] | (b) Zeng, Ch. F.; He, Y.; Li, Q.; Dong, L. Chin. J. Org. Chem. 2023, 43, 1115. (in Chinese) |
| [1] | (曾成富, 何媛, 李清, 董琳, 有机化学, 2023, 43, 1115.) |
| [1] | (c) Yin, J. W.; Zhang, J. Y.; Cai, C. Q.; Deng, G. J.; Gong, H. Org. Lett. 2019, 21, 387. |
| [1] | (d) Zheng, Y. L.; Newman, S. G. ACS Catal. 2019, 9, 4426. |
| [1] | (e) Dong, H.; Hou, M. F. Chin. J. Org. Chem. 2017, 37, 267. (in Chinese) |
| [1] | (董浩, 侯梅芳, 有机化学, 2017, 37, 267.) |
| [1] | (f) Wang, J.; Ren, J. M.; Zhu, Y. P.; Sun, X. Q.; Hu, P. F.; Mu, X.; Zeng, B. B. Tetrahedron Lett. 2023, 116, 154312. |
| [1] | (g) Tian, Q. Q.; Gan, Z. J.; Wang, X. T.; Li, D.; Luo, W.; Wang, H. J.; Dai, Z. S.; Yuan, J. Y. Molecules 2018, 23, 2234. |
| [1] | (h) Ye, D. F.; Chen, H.; Liu, Z. Y.; Lei, C. H. Chin. J. Org. Chem. 2021, 41, 1658. (in Chinese) |
| [1] | (叶丹锋, 陈浩, 刘志园, 雷川虎, 有机化学, 2021, 41, 1658.) |
| [1] | (i) Lanigan, R. M.; Starkov, P.; Sheppard, T. D. J. Org. Chem. 2013, 78, 4512. |
| [2] | (a) Walsh, C. T.; O’Brien, R. V.; Khosla, C. Angew. Chem., Int. Ed. 2013, 52, 7098. |
| [2] | (b) Todorovic, M.; Perrin, D. M. Pept. Sci. 2020, 17, 3895. |
| [2] | (c) Sheng, G.Z.; Zhang, W. Chin. J. Org. Chem. 2013, 33, 2271. (in Chinese) |
| [2] | (盛国柱, 张炜, 有机化学, 2013, 33, 2271.) |
| [2] | (d) Chapman, R. S. L.; Lawrence, R.; Williams, J. M. J.; Bull, S. D. Org. Lett. 2017, 19, 4908. |
| [2] | (e) Gerack, C. J.; McElwee-White, L. Molecules 2014, 19, 7689. |
| [3] | (a) Li, Z. Y.; Chen, Y. Z.; Wan, N.W. Chin. J. Synth. Chem. 2022, 30, 419. (in Chinese) |
| [3] | (李正一, 陈永正, 万南微, 合成化学, 2022, 30, 419.) |
| [3] | (b) Chen, J.; Jia, J.; Guo, Z.; Zhang, J.; Xie, M. Tetrahedron Lett. 2019, 60, 1426. |
| [3] | (c) Tan, C.; Liu, Y. G.; Liu, X. Y.; Jia, H. X.; Xu, K.; Huang, S. H.; Wang, J. W.; Tan, J. J. Org. Chem. Front. 2020, 7, 780. |
| [4] | (a) Reddy, T. N.; Beatriz, A.; Rao, V. J.; Lima, D. P. Chem.-Asian J. 2019, 14, 344. |
| [4] | (b) Lundberg, H.; Tinnis, F.; Selander, N.; Adolfsson, H. Chem. Soc. Rev. 2014, 43, 2714. |
| [4] | (c) Pattabiraman, V. R.; Bode, J. W. Nature 2011, 480, 471. |
| [4] | (d) Yan, Y. Z.; Niu, B.; Xu, K.; Yu, J. H.; Zhi, H. H.; Liu, Y. Q. Adv. Synth. Catal. 2016, 358, 212. |
| [5] | Hinz, B.; Cheremina, O.; Brune, K. FASEB J. 2008, 22, 383. |
| [6] | Perez-Aso, M.; Montesinos, M. C.; Mediero, A.; Wilder, T.; Schafer, P. H. Arthritis Res. Ther. 2015, 17, 249. |
| [7] | Abe, H.; Kikuchi, S.; Hayakawa, K.; Iida, T.; Nagahashi, N.; Maeda, K. ACS Med. Chem. Lett. 2011, 2, 320. |
| [8] | Primiano, G.; Vollono, C.; Dono, F.; Servidei, S. Epilepsy Res. 2018, 139, 135. |
| [9] | Mahesh, S.; Tang, K. C.; Raj, M. Molecules 2018, 23, 2615. |
| [10] | Young, I. S.; Glass, A. L.; Cravillion, T.; Han, C.; Zhang, H.; Gosselin, F. Org. Lett. 2018, 20, 3902. |
| [11] | Dander, J. E.; Baker, E. L.; Garg, N. K. Chem. Sci. 2017, 8, 6433. |
| [12] | Pelletier, G.; Powell, D. A. Org. Lett. 2006, 8, 6031. |
| [13] | Shekhar, A. C.; Kumar, A. R.; Sathaiah, G.; Paul, V. L.; Sridhar, M.; Rao, P. S. Tetrahedron Lett. 2009, 50, 7099. |
| [14] | (a) Li, Y.; Jia, F.; Li, Z. Chem.-Eur. J. 2013, 19, 82. |
| [14] | (b) Becerra-Figueroa, L.; Ojeda-Porras, A.; Gamba-Sanchez, D. J. Org. Chem. 2014, 79, 4544. |
| [15] | Bon, E.; Bigg, D. C. H.; Bertrand, G. J. Org. Chem. 1994, 59, 4035. |
| [16] | Jiang, H.; Hu, Z.; Gan, C.; Sun, B.; Kong, S.; Bian, F. J. Mol. Catal. 2021, 504, 111490. |
| [17] | Shah, N.; Gravel, E.; Jawale, D. V.; Doris, E.; Namboothiri, I. N. N. ChemCatChem 2014, 6, 2201. |
| [18] | Zhang, L.; Han, Z.; Zhao, X.; Wang, Z.; Ding, K. Angew. Chem., Int. Ed. 2015, 54, 6186. |
| [19] | Lenstra, D. C.; Nguyen, D. T.; Mecinovi?, J. Tetrahedron, 2015, 71, 5547. |
| [20] | Kong, X.; Xu, B. Org. Lett. 2018, 20, 4495. |
| [21] | Pathare, S. P.; Jain, A. K. H.; Akamanchi, K. G. RSC Adv. 2013, 21, 7697. |
| [22] | Rasheed, S.; Rao, D. N.; Reddy, A. S.; Shankar, R.; Das, P. RSC Adv. 2015, 5, 10567. |
| [23] | Karami, B.; Farahi, M.; Pam, F. Tetrahedron Lett. 2014, 55, 6292. |
| [24] | Allen, C. L.; Atkinson, B. N.; Williams, J. M. J. Angew. Chem., Int. Ed. 2012, 51, 1383. |
| [25] | Laconde, G.; Amblard, M.; Martinez, J. Eur. J. Org. Chem. 2019, 2019, 85. |
| [26] | Sonawane, R. B.; Rasal, N. K.; Jagtap, S. V. Org. Lett. 2017, 19, 2078. |
| [27] | Sonawane, R. B.; Rasal, N. K.; Bhange, D. S.; Jagtap, S. V. ChemCatChem 2018, 10, 3907. |
| [28] | (a) Kumar, V.; Dhawan, S.; Girase, P. S.; Singh, P.; Karpoormath, R. Eur. J. Org. Chem. 2021, 2021, 5627. |
| [28] | (b) Girase, P. S.; Kumar, V.; Dhawan, S.; Karpoormath, R. ChemistrySelect 2022, 7, 3237. |
| [28] | (c) Acosta-Guzmán, P.; Mateus-Gómez, A.; Gamba-Sánchez, D. Molecules 2018, 23, 2382. |
| [28] | (d) Yan, Y. Z.; Cui, C.; Li, Z. Chin. J. Org. Chem. 2018, 38, 2501. (in Chinese) |
| [28] | (闫溢哲, 崔畅, 李政, 有机化学, 2018, 38, 2501.) |
| [29] | Rao, S. N.; Mohan, D. C.; Adimurthy, S. Tetrahedron 2016, 72, 4889. |
| [30] | Mahajan, S.; Slathia, N.; Kapoor, K. K. Tetrahedron Lett. 2020, 61, 151859. |
| [31] | Zhou, Z. J.; Kweon, J.; Jung, H.; Kim, D.; Seo, S.; Chang, S. J. Am. Chem. Soc. 2022, 144, 9161. |
| [32] | Lakshmi, V. M.; Hsu, F. F.; Davis, B. B.; Zenser, T. V. Chem. Res. Toxicol. 2001, 14, 312. |
| [33] | Wang, J. K.; Zong, Y. X.; Wang, X. C.; Hu, Y. L.; Yue, G. R. Chin. Chem. Lett. 2015, 26, 1376. |
/
| 〈 |
|
〉 |