

碘介导下通过氧化性C—C键形成合成β-硝基胺与α-胺基腈类化合物
收稿日期: 2023-05-15
修回日期: 2023-06-23
网络出版日期: 2023-07-06
基金资助
河南省疫情防控应急科研攻关(221111311400); 河南省高等学校青年骨干教师培养计划(2021GGJS012); 国家自然科学基金(82130103)
Synthesis of β-Nitroamines and α-Aminonitriles by I2-Mediated Oxidative C—C Bond Formation
Received date: 2023-05-15
Revised date: 2023-06-23
Online published: 2023-07-06
Supported by
Henan Province Epidemic Prevention and Control Emergency Research Project(221111311400); Young Backbone Teachers Fund of Henan Province(2021GGJS012); National Natural Science Foundation of China(82130103)
李倩敏 , 王漫漫 , 于文全 , 常俊标 . 碘介导下通过氧化性C—C键形成合成β-硝基胺与α-胺基腈类化合物[J]. 有机化学, 2023 , 43(11) : 3966 -3976 . DOI: 10.6023/cjoc202305021
A transition-metal-free C—C bond formation reaction is developed employing molecular iodine as the sole oxidant to access β-nitroamines, α-aminonitriles and α-aminophosphonates from readily accessible tertiary amines with nitroalkanes, trimethylsilyl cyanide and phosphite, respectively. The present synthetic approach is operationally simple, has a broad substrate scope, and can be successfully conducted on a gram scale.
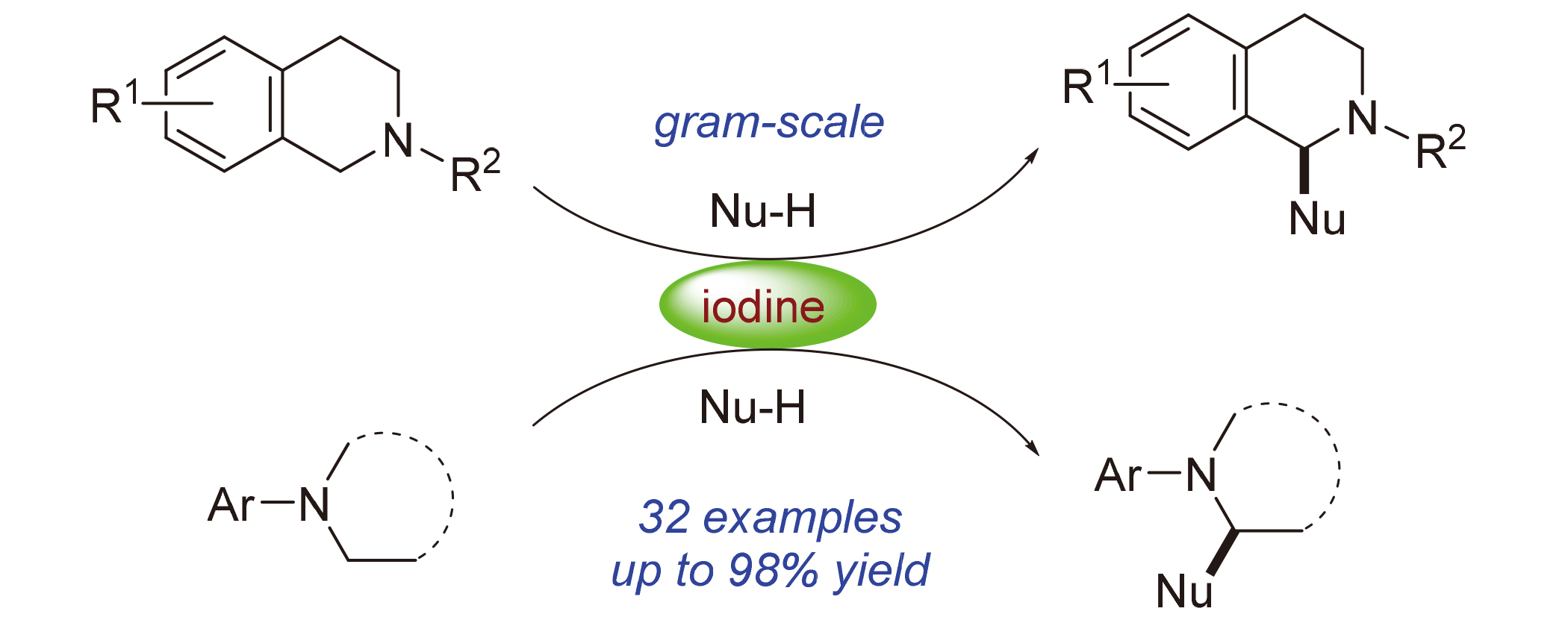
Key words: iodine; C—C bond formation; tertiary amine; β-nitroamine; α-aminonitrile
| [1] | (a) Tan, J.-P.; Li, X.; Chen, Y.; Rong, X.; Zhu, L.; Jiang, C.; Xiao, K.; Wang, T. Sci. China Chem. 2020, 63, 1091. |
| [1] | (b) El Sayed, M. T.; Sarhan, A. E.; Ahmed, E.; Khattab, R. R.; Elnaggar, M.; El-Messery, S. M.; Shaldam, M. A.; Hassan, G. S. ChemistrySelect 2020, 5, 3445. |
| [2] | (a) Méndez-álvarez, E.; Soto-Otero, R.; Sánchez-Sellero, I.; Lamas, M. L.-R. Life Sci. 1997, 60, 1719. |
| [2] | (b) Deng, X.; Lin, F.; Zhang, Y.; Li, Y.; Zhou, L.; Lou, B.; Li, Y.; Dong, J.; Ding, T.; Jiang, X.; Wang, R.; Ye, D. Eur. J. Med. Chem. 2014, 73, 1. |
| [2] | (c) Yang, R.; Ruan, Q.; Zhang, B.-Y.; Zheng, Z.-L.; Miao, F.; Zhou, L.; Geng, H.-L. Molecules 2014, 19, 8051. |
| [2] | (d) Cao, F.-J.; Xu, M.-X.; Zhou, B.-H.; Du, Y.-S.; Yao, J.-H.; Zhou, L. Toxicol. In Vitro 2019, 54, 295. |
| [2] | (e) Gajic, M.; Ilic, B. S.; Bondzic, B. P.; Dzambaski, Z.; Kojic, V. V.; Jakimov, D. S.; Kocic, G.; Smelcerovic, A. Chem. Biodiversity 2021, 18, e2100261. |
| [3] | Pabu??uoglu, V.; Arar, G.; G?zler, T.; Freyer, A. J.; Shamma, M. J. Nat. Prod. 1989, 52, 716. |
| [4] | Romo-Pérez, A.; Miranda, L. D.; Chávez-Blanco, A. D.; Due?as- González, A.; Camacho-Corona, M. d. R.; Acosta-Huerta, A.; García, A. Eur. J. Med. Chem. 2017, 138, 1. |
| [5] | Hayashi, K.; Minoda, K.; Nagaoka, Y.; Hayashi, T.; Uesato, S. Bioorg. Med. Chem. Lett. 2007, 17, 1562. |
| [6] | Cao, F.-J.; Yang, R.; Lv, C.; Ma, Q.; Lei, M.; Geng, H.-L.; Zhou, L. Eur. J. Pharm. Sci. 2015, 67, 45. |
| [7] | (a) Dyker, G. Angew. Chem. Int. Ed. 1997, 36, 1700. |
| [7] | (b) Ballini, R.; Petrini, M. Tetrahedron 2004, 60, 1017. |
| [7] | (c) Bernardi, L.; Bonini, B. F.; Capitó, E.; Dessole, G.; Comes-Franchini, M.; Fochi, M.; Ricci, A. J. Org. Chem. 2004, 69, 8168. |
| [7] | (d) Murahashi, S.-I.; Zhang, D. Chem. Soc. Rev. 2008, 37, 1490. |
| [8] | (a) Tsang, A. S.-K.; Todd, M. H. Tetrahedron Lett. 2009, 50, 1199. |
| [8] | (b) Nobuta, T.; Fujiya, A.; Yamaguchi, T.; Tada, N.; Miura, T.; Itoh, A. RSC Adv. 2013, 3, 10189. |
| [8] | (c) Liu, P.-Y.; Zhang, C.; Zhao, S.-C.; Yu, F.; Li, F.; He, Y.-P. J. Org. Chem. 2017, 82, 12786. |
| [8] | (d) Zhang, R.; Qin, Y.; Zhang, L.; Luo, S. J. Org. Chem. 2019, 84, 2542. |
| [9] | (a) Chu, L.; Qing, F.-L. Chem. Commun. 2010, 46, 6285. |
| [9] | (b) Zhang, Y.; Peng, H.; Zhang, M.; Cheng, Y.; Zhu, C. Chem. Commun. 2011, 47, 2354. |
| [9] | (c) Kumar, R. A.; Saidulu, G.; Prasad, K. R.; Kumar, G. S.; Sridhar, B.; Reddy, K. R. Adv. Synth. Catal. 2012, 354, 2985. |
| [9] | (d) Nobuta, T.; Tada, N.; Fujiya, A.; Kariya, A.; Miura, T.; Itoh, A. Org. Lett. 2013, 15, 574. |
| [9] | (e) Zhu, S.-L.; Ou, S.; Zhao, M.; Shen, H.; Wu, C.-D. Dalton Trans. 2015, 44, 2038. |
| [9] | (f) Liang, W.; Zhang, T.; Liu, Y.; Huang, Y.; Liu, Z.; Liu, Y.; Yang, B.; Zhou, X.; Zhang, J. ChemSusChem 2018, 11, 3586. |
| [10] | (a) Condie, A. G.; González-Gómez, J. C.; Stephenson, C. R. J. J. Am. Chem. Soc. 2010, 132, 1464. |
| [10] | (b) Rueping, M.; Zhu, S.; Koenigs, R. M. Chem. Commun. 2011, 12709. |
| [10] | (c) Gandy, M. N.; Raston, C. L.; Stubbs, K. A. Chem. Commun. 2015, 51, 11041. |
| [10] | (d) Wang, X.-Z.; Meng, Q.-Y.; Zhong, J.-J.; Gao, X.-W.; Lei, T.; Zhao, L.-M.; Li, Z.-J.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Chem. Commun. 2015, 51, 11256. |
| [10] | (e) Li, X.; Li, Y.; Huang, Y.; Zhang, T.; Liu, Y.; Yang, B.; He, C.; Zhou, X.; Zhang, J. Green Chem. 2017, 19, 2925. |
| [10] | (f) Chen, K.; Cheng, Y.; Chang, Y.; Li, E.; Xu, Q.-L.; Zhang, C.; Wen, X.; Sun, H. Tetrahedron 2018, 74, 483. |
| [10] | (g) Ide, T.; Shimizu, K.; Egami, H.; Hamashima, Y. Tetrahedron Lett. 2018, 59, 3258. |
| [10] | (h) Kosso, A. R. O.; Sellet, N.; Baralle, A.; Cormier, M.; Goddard, J.-P. Chem. Sci. 2021, 12, 6964. |
| [10] | (i) Lin, C.; Li, P.; Wang, L. Tetrahedron Lett. 2021, 73, 153102. |
| [11] | (a) Baslé, O.; Li, C.-J. Green Chem. 2007, 9, 1047. |
| [11] | (b) Murahashi, S.-I.; Nakae, T.; Terai, H.; Komiya, N. J. Am. Chem. Soc. 2008, 130, 11005. |
| [11] | (c) Alagiri, K.; Prabhu, K. R. Org. Biomol. Chem. 2012, 10, 835. |
| [11] | (d) Meng, Q.-Y.; Liu, Q.; Zhong, J.-J.; Zhang, H.-H.; Li, Z.-J.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Org. Lett. 2012, 14, 5992. |
| [11] | (e) Brzozowski, M.; Forni, J. A.; Savage, G. P.; Polyzos, A. Chem. Commun. 2015, 51, 334. |
| [11] | (f) Patil, M. R.; Dedhia, N. P.; Kapdi, A. R.; Kumar, A. V. J. Org. Chem. 2018, 83, 4477. |
| [11] | (g) Wang, H.; Wang, A.; Xia, Z.; Zhou, W.; Sun, Z.; Qian, J.; He, M. Chin. J. Org. Chem. 2020, 40, 2099. (in Chinese) |
| [11] | (王慧, 王安玮, 夏珍珍, 周维友, 孙中华, 钱俊峰, 何明阳, 有机化学, 2020, 40, 2099.) |
| [11] | (h) Bjerg, E. E.; Marchan-Garcia, J.; Buxaderas, E.; Moglie, Y.; Radivoy, G. J. Org. Chem. 2022, 87, 13480. |
| [12] | Tanoue, A.; Yoo, W.-J.; Kobayashi, S. Org. Lett. 2014, 16, 2346. |
| [13] | Dhineshkumar, J.; Lamani, M.; Alagiri, K.; Prabhu, K. R. Org. Lett. 2013, 15, 1092. |
| [14] | (a) Liu, J.; Wei, W.; Zhao, T.; Liu, X.; Wu, J.; Yu, W.; Chang, J. J. Org. Chem. 2016, 81, 9326. |
| [14] | (b) Lv, Z.; Wang, B.; Hu, Z.; Zhou, Y.; Yu, W.; Chang, J. J. Org. Chem. 2016, 81, 9924. |
| [15] | (a) Yi, X.; Zhao, Z.; Wang, M.; Yu, W.; Chang, J. Org. Lett. 2022, 24, 8703. |
| [15] | (b) Wang, M.; Ye, W.; Sun, N.; Yu, W.; Chang, J. J. Org. Chem. 2023, 88, 1061. |
| [16] | (a) Jayram, J.; Xulu, B. A.; Jeena, V. Tetrahedron 2019, 75, 130617. |
| [16] | (b) Huang, H.-Y.; Wu, H.-R.; Wei, F.; Wang, D.; Liu, L. Org. Lett. 2015, 17, 3702. |
| [16] | (c) Gromada, J.; Matyjaszewski, K. Macromolecules 2001, 34, 7664. |
| [16] | (d) Wan, J.-P.; Zhong, S.; Guo, Y.; Wei, L. Eur. J. Org. Chem. 2017, 440. |
| [17] | Tran, V. H.; La, M. T.; Kim, H.-K. Tetrahedron Lett. 2019, 60, 1860. |
| [18] | Yan, C.; Liu, Y.; Wang, Q. RSC Adv. 2014, 4, 60075. |
| [19] | Zhang, L.; Peng, C.; Zhao, D.; Wang, Y.; Fu, H.-J.; Shen, Q.; Li, J.-X. Chem. Commun. 2012, 48, 5928. |
| [20] | Huang, B.-Q.; Chen, Y.; Zhang, X.-J.; Yan, M. Eur. J. Org. Chem. 2021, 3015. |
| [21] | Tian, H.; Xu, W.; Liu, Y.; Wang, Q. Chem. Commun. 2019, 55, 14813. |
| [22] | Qian, W.; Zhou, X. Chinese J. Org. Chem. 2013, 33, 2430. |
| [23] | Tran, V. H.; La, M. T.; Kang, S.; Kim, H.-K. Org. Biomol. Chem. 2020, 18, 5008. |
| [24] | Matsuda, N.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem. Int. Ed. 2012, 124, 3702. |
| [25] | Zhou, J.; Li, L.; Wang, S.; Yan, M.; Wei, W. Green Chem. 2020, 22, 3421. |
| [26] | Tsang, A. S.-K.; Hashmi, A. S. K.; Comba, P.; Kerscher, M.; Chan, B.; Todd, M. H. Chem.-Eur. J. 2017, 23, 9313. |
| [27] | Kim, H.-K.; Lee, A. Tetrahedron Lett. 2016, 57, 4890. |
| [28] | Casarini, D.; Davalli, S.; Lunazzi, L. J. Org. Chem. 1989, 54, 4616. |
| [29] | Shu, X.-Z.; Xia, X.-F.; Yang, Y.-F.; Ji, K.-G.; Liu, X.-Y.; Liang, Y.-M. J. Org. Chem. 2009, 74, 7464. |
| [30] | Zhu, S.-S.; Liu, Y.; Chen, X.-L.; Qu, L.-B.; Yu, B. ACS Catal. 2022, 12, 126. |
| [31] | Yu, J.; Wang, Z.; Zhang, Y.; Su, W. Tetrahedron 2015, 71, 6116. |
| [32] | Zhang, G.; Ma, Y.; Cheng, G.; Liu, D.; Wang, R. Org. Lett. 2014, 16, 656. |
| [33] | Wang, J.-H.; Li, X.-B.; Li, J.; Lei, T.; Wu, H.-L.; Nan, X.-L.; Tung, C.-H.; Wu, L.-Z. Chem. Commun. 2019, 55, 10376. |
| [34] | Liu, L.; Wang, Z.; Fu, X.; Yan, C.-H. Org. Lett. 2012, 14, 5692. |
| [35] | Xia, Q.; Zhang, W.; Li, Y.; Cheng, L.; Liang, X.; Dai, P. CN 112390696B, 2021. |
| [36] | Mudithanapelli, C.; Dhorma, L. P.; Kim, M.-H. Org. Lett. 2019, 21, 3098. |
/
| 〈 |
|
〉 |