

无过渡金属条件下二芳基硒化合物的合成
收稿日期: 2023-03-06
修回日期: 2023-05-29
网络出版日期: 2023-07-27
基金资助
国家自然科学基金(22074095); “十三五”期间北京市属高校高水平教师(CIT&TCD20190330)
Synthesis of Diaryl Selenium Compounds without Transition-Metal Catalyst
Received date: 2023-03-06
Revised date: 2023-05-29
Online published: 2023-07-27
Supported by
National Natural Science Foundation of China(22074095); High-Level Teachers in Beijing Municipal Universities in the Period of 13th Five Year Plan(CIT&TCD20190330)
孙婧 , 张萌萌 , 锅小龙 , 王琪 , 王陆瑶 . 无过渡金属条件下二芳基硒化合物的合成[J]. 有机化学, 2023 , 43(12) : 4251 -4260 . DOI: 10.6023/cjoc202303011
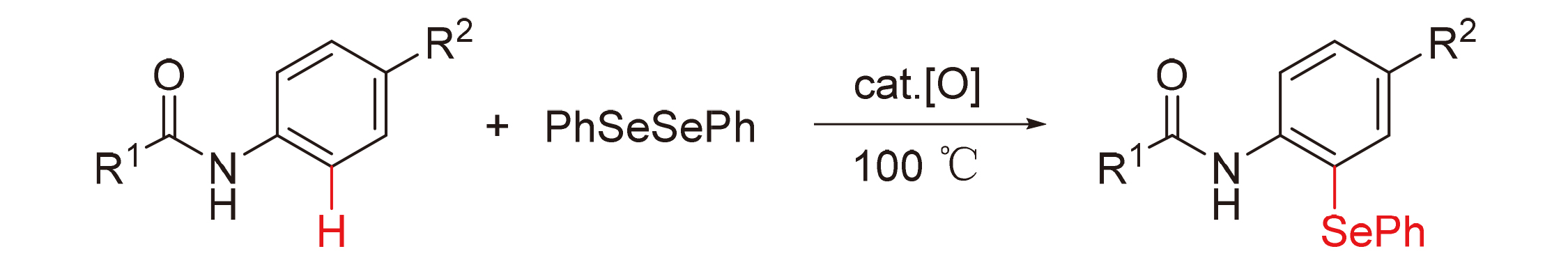
A series of diaryl selenium compounds were synthetized from N-arylamide compounds and diphenyl diselenide, using K2S2O8 as oxidant without any transition metal catalyst. Mechanistic studies reveal that the reaction proceeds a single-electron transfer radical process. The dominant products are o-substituted and p-substituted product, depending on the stability of intermediates. The synthetic reaction exhibits some outstanding advantages, such as substrate tolerance, high regioselectivity, convenience and low-price.
| [1] | Guo T.; Dong Z; Zhang P.; Xing W.; Li L. Tertahedron Lett. 2018, 59, 2554. |
| [2] | Vieira B. M.; Thurow S.; Costa M.; Casaril A. M.; Domingues M.; Schumacher R. F.; Perin G.; Alves D.; Savegnago L.; Lenard? E. J. Asian J. Org. Chem. 2017, 6, 1635. |
| [3] | Zhang J. D.; Zhan Y.; Li H. Y. W.; Qi Y.; Wang R. P.; Meng L. Chin. J. Org. Chem. 2020, 40, 1847. (in Chinese) |
| [3] | (张继东, 詹妍, 李胡月雯, 齐怡, 王瑞鹏, 孟莉, 有机化学, 2020, 40, 1847.) |
| [4] | Lou Z.; Li P.; Han K. Acc. Chem. Res. 2015, 48, 1358. |
| [5] | Wen Z. Y.; Xu J. W.; Wang Z. W.; Qi H.; Xu Q. L.; Bai Z. S.; Zhang Q.; Bao K.; Wu Y. L.; Zhang W. G. Eur. J. Med. Chem., 2015, 90, 184. |
| [6] | Engman L.; Stern D.; Frisell H.; Vessman K.; Berglund M.; Ek B.; Andersson C. M. Bioorg. Med. Chem. 1995, 3, 1255. |
| [7] | Guan Q.; Han C. M.; Zuo D. Y.; Zhai M. A.; Li Z. Q.; Zhang Q.; Zhai Y. P.; Jiang X. W.; Bao K.; Wu Y. L.; Zhang W. G. Eur. J. Med. Chem. 2014, 87, 306. |
| [8] | Manjare S. T.; Kim Y.; Churchill D. G. Acc. Chem. Res. 2014, 47, 2985. |
| [9] | (a) Reddy V. P.; Kumar A. V.; Swapna K.; Rao K. R. Org. Lett. 2009, 11, 951. |
| [9] | (b) Halle M. B.; Yudhistira T.; Lee W. H.; Mulay S. V.; Churchill D. G. Org. Lett. 2018, 20, 3557. |
| [9] | (c) Jones K. D.; Power D. J.; Bierer D.; Gericke K. M.; Stewart S. G. Org. Lett. 2018, 20, 208. |
| [9] | (d) Lee S. C.; Liao H.-H.; Chatupheeraphat A.; Rueping M. Chem. Eur. J. 2018, 24, 3608. |
| [9] | (e) Zhang J. R.; Zhan L. Z.; Wei L.; Ning Y.-Y.; Zhong X. L.; Lai J. X.; Xu L.; Tang R. Y. Adv. Synth. Catal. 2018, 360, 533. |
| [9] | (f) Gao C.; Wu G.; Min L.; Liu M.; Gao W.; Ding J.; Chen J.; Huang X.; Wu H. J. Org. Chem. 2017, 82, 250. |
| [10] | (a) Sun K.; Wang X.; Li C.; Wang H.; Li L. Org. Chem. Front. 2020, 7, 3100. |
| [10] | (b) Wang X.; Wang Z. C.; Li Z. J.; Sun K. Chin. Chem. Lett. 2023, 34, 108045. |
| [10] | (c) Wang P.; Tang S.; Huang P.; Lei A. Angew. Chem., Int. Ed. 2017, 56, 3009. |
| [10] | (d) Hostier T.; Ferey V.; Ricci G.; Pardo D. G.; Cossy J. Org. Lett. 2015, 17, 3898. |
| [11] | (a) Iwasaki M.; Tsuchiya Y.; Nakajima K.; Nishihara Y. Org. Lett. 2014, 16, 4920. |
| [11] | (b) Iwasaki M.; Kaneshika W.; Tsuchiya Y.; Nakajima K.; Nishihara Y. J. Org. Chem. 2014, 79, 11330. |
| [11] | (c) Mandal A.; Sahoo H.; Baidya M. Org. Lett. 2016, 18, 3202. |
| [11] | (d) Singh B. K.; Bairy G.; Jana R. ChemistrySelect 2017, 2, 9227 |
| [12] | (a) Sun M. Y.; Xu K.; Guo B.-B.; Zeng C.-C. Chin. J. Org. Chem. 2021, 41, 2302. (in Chinese) |
| [12] | (孙名扬, 徐坤, 郭兵兵, 曾程初, 有机化学, 2021, 41, 2302.) |
| [12] | (b) Ma W. B.; Weng Z.; Rogge T.; Gu L.; Lin J.; Peng A.; Luo X.; Gou X.; Ackermann L. Adv. Synth. Catal. 2018, 360, 704. |
| [13] | Ma W. B.; Weng Z.; Torben R.; Gu L.; Lin J. Adv. Synth. Catal. 2018, 360, 704. |
| [14] | Wang K.; Hou J. H.; Zhang C. J.; Cheng K.; Bai R.-R.; Xie Y.-Y. Adv. Synth. Catal. 2020, 362, 2947. |
| [15] | Liu Q.; Zhang Q. B. Green Chem. 2017, 19, 5559. |
| [16] | Lazzaris M. J.; Martins G. M.; Xavier F. R.; Braga A. L.; Mendes S. R. Eur. J. Org. Chem. 2021, 31, 4411. |
| [17] | Lan J. Y.; Xie H. S.; Lu X.-X.; Deng Y. F.; Jiang H. F.; Zeng W. Org. Lett. 2017, 19, 4279. |
| [18] | Zhou J.; Li B.; Qian Z. C.; Shi B. F. Adv. Synth. Catal. 2014, 356, 1038. |
/
| 〈 |
|
〉 |