

含双硫脲基团分子钳在非极性溶剂中识别中性分子
收稿日期: 2023-06-18
修回日期: 2023-07-21
网络出版日期: 2023-08-30
基金资助
国家自然科学基金(21961042); 及玉林师范学院科研(G2021ZK16); 玉林师范学院高层次人才(G2023ZK12); 及玉林师范学院科研(S202210606118)
Recognition of Bis-thiourea Tweezers to Neutral Molecules in Non-Polar Solvent
Received date: 2023-06-18
Revised date: 2023-07-21
Online published: 2023-08-30
Supported by
National Natural Science Foundation of China(21961042); Program of Yulin Normal University(G2021ZK16); Yulin Normal University High-Level Talent Project(G2023ZK12); Program of Yulin Normal University(S202210606118)
陈珊 , 陈志林 , 胡琼 , 蒙艳双 , 黄悦 , 陶萍芳 , 卢丽如 , 黄国保 . 含双硫脲基团分子钳在非极性溶剂中识别中性分子[J]. 有机化学, 2024 , 44(1) : 277 -281 . DOI: 10.6023/cjoc202306013
Three different molecular tweezers containing bis-thiourea groups were efficiently synthesized by a series of organic reactions using anthracene as raw materials. Their structures were confirmed and characterized by melting point, 1H NMR, 13C NMR and HRMS. The recognition behavior of molecular tweezers to several neutral molecules in non-polar environment was studied in detail by fluorescence spectroscopy. The results show that the molecular tweezer 9,10-bis- trifluoromethylphenyl-thioureamethyl anthracene (T-3) has good recognition ability for several guest molecules, and the synergistic effect between the hydrogen bonding and multiple C—H…π interactions on molecular recognition was discussed.
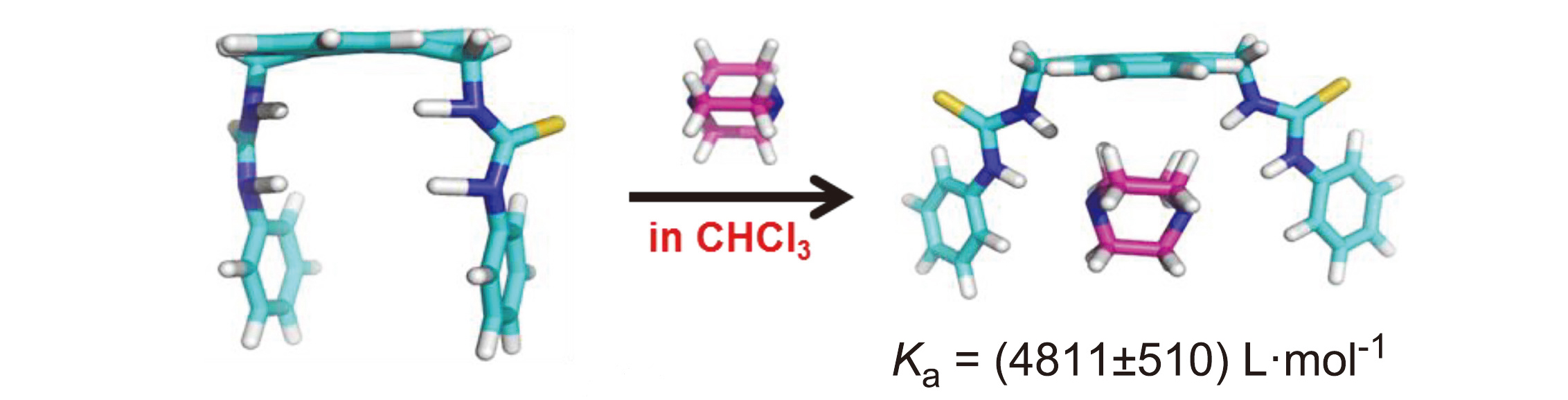
Key words: bis-thiourea tweezer; synthesis; recognition; neutral molecule
| [1] | Berthod, A. Anal. Chem. 2006, 78, 2093. |
| [2] | Pedersen, C. J. J. Am. Chem. Soc. 1967, 89, 7017. |
| [3] | Zhao, Z.-G.; Liu, X.-L.; Li, Q.-H.; Chen, S.-H. Chin. J. Org. Chem. 2009, 29, 1336 (in Chinese). |
| [3] | (赵志刚, 刘兴利, 李清寒, 陈淑华, 有机化学, 2009, 29, 1336.) |
| [4] | Salonen, L. M.; Ellermann, M.; Diederich, F. Angew. Chem., Int. Ed. 2011, 50, 4808. |
| [5] | Chen, C.-W.; Whitlock Jr., H. W. J. Am. Chem. Soc. 1978, 100, 4921. |
| [6] | Hardouin-Lerouge, M.; Hudhomme, P.; Salle, M. Chem. Soc. Rev. 2001, 40, 30. |
| [7] | Legouin, B.; Uriac, P.; Tomasi, S.; Toupet, L.; Bondon, A.; Vande Weghe, P. Org. Lett. 2009, 11, 745. |
| [8] | Colquhoun, H. M.; Zhu, Z.; Williams, D. J. Org. Lett. 2003, 5, 4353. |
| [9] | Canevet, D.; Sallé, M.; Zhang, G.; Zhang, D.; Zhu, D. Chem. Commun. 2009, 2245. |
| [10] | (a) Park, J.-S.; Le Derf, F.; Bejger, C.-M.; Lynch, V.-M.; Sessler, J.-L.; Nielsen, K.-A.; Johnsen, C.; Jeppesen, J.-O. Chem.-Eur. J. 2010, 16, 848. |
| [10] | (b) Dolensky, B.; Havlík, M.; Král, V. Chem. Soc. Rev. 2012, 41, 3839. |
| [10] | (c) Zimmerman, S. Beilstein J. Org. Chem. 2016, 12, 125. |
| [10] | (d) Kumar, R.; Srivastava, A. Chem.-Eur. J. 2016, 22, 3224. |
| [10] | (e) Fu, T.; Han, Y.; Ao, L.; Wang, F. Organometallics 2016, 35, 2850. |
| [10] | (f) Tsuchido, Y.; Suzaki, Y.; Ide, T.; Osakada, K. Chem.-Eur. J. 2014, 20, 4762. |
| [11] | (a) Gunnlaugsson, T.; Davis, A. P.; O'Briena, J. E.; Glynna, M. Org. Biomol. Chem. 2005, 3, 48. |
| [11] | (b) Zhang, Z.-Y.; Li, C. Acc. Chem. Res. 2022, 55, 916. |
| [11] | (c) Zhang, X; Wang, X.; Wang, B.; Ding, Z.; Li, C. Chin. Chem. Lett. 2020, 31, 3230. |
| [12] | (a) Huang, G.; He, Z.; Cai, C.; Pan, F.; Yang, D.; Rissanen, K.; Jiang, W. Chem. Commun. 2015, 15, 15490. |
| [12] | (b) Huang, G.; Valkonen, A.; Rissanen, K.; Jiang, W. Chem. Commun. 2016, 52, 9078. |
| [13] | Altava, B.; Burguete, M. I.; Escuder, B.; Luis, S. V.; García-Espa?a, E.; Mu?oz, M. C. Tetrahedron 1997, 53, 2629. |
/
| 〈 |
|
〉 |