

取代基电性效应对碳硅还原消除区域选择性调控的理论研究
收稿日期: 2023-08-11
修回日期: 2023-08-29
网络出版日期: 2023-08-31
基金资助
国家自然科学基金(22003006); 国家自然科学基金(22201027); 国家自然科学基金(22271034); 中国博士后科学基金(2021M700578)
Theoretical Study of How Electronic Effect of Substituent Affects Regioselectivity of C—Si Reductive Elimination
Received date: 2023-08-11
Revised date: 2023-08-29
Online published: 2023-08-31
Supported by
National Natural Science Foundation of China(22003006); National Natural Science Foundation of China(22201027); National Natural Science Foundation of China(22271034); China Postdoctoral Science Foundation(2021M700578)
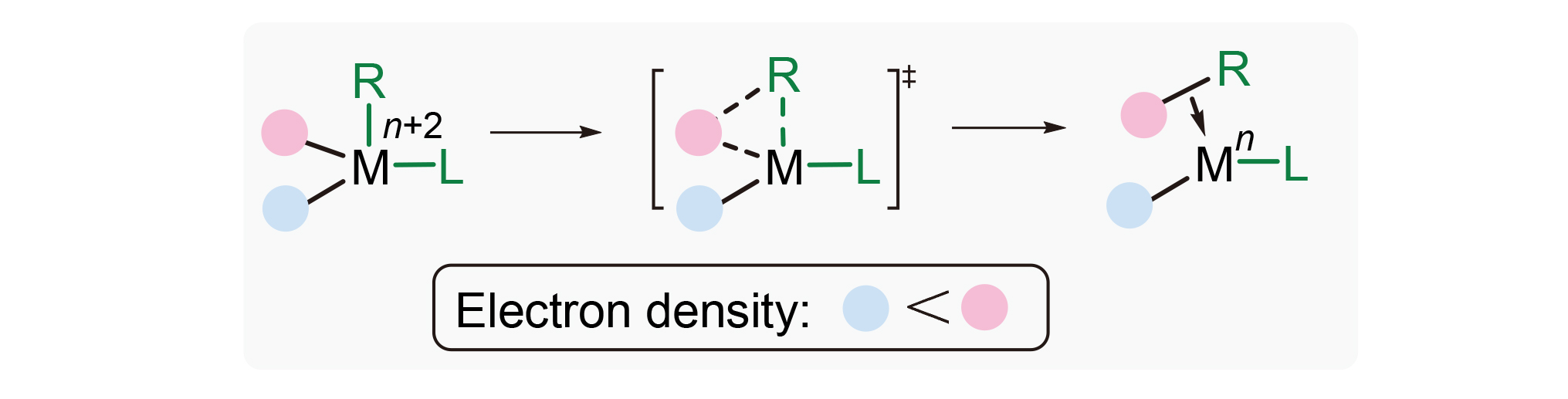
有机反应区域选择性的调控是有机化学的重要研究内容之一, 而电性效应则是其重要调控因素. 运用密度泛函理论计算, 以钯催化2-碘联苯化合物与硅杂环丁烷的环化反应为模板, 研究了取代基电性效应在还原消除过程中对区域选择性的影响, 并给出了该反应的详细反应机理. 计算结果表明, 该反应经历了Pd—I键氧化加成、协同金属去质子、Pd—Si键氧化加成、还原消除过程得到硅杂八元环产物, 且C—Si键还原消除是反应的速率决定步骤. 对Pd(IV)还原消除过渡态中电子效应的研究证明, 当使用不对称2-碘联苯作为反应底物时, 芳环电子密度是区域选择性的主要控制因素, 电子密度更高的基团更容易发生还原消除, 与该基元反应电子流向一致.
彭菊 , 何晓倩 , 廖黎丽 , 白若鹏 , 蓝宇 . 取代基电性效应对碳硅还原消除区域选择性调控的理论研究[J]. 有机化学, 2023 , 43(10) : 3608 -3613 . DOI: 10.6023/cjoc202308009
Regioselectivity adjustment is one of the key research fields in organic chemistry, and electronic effect is the key factor for adjustment. In this work, density functional theory (DFT) calculation was carried out to investigate how electronic effect of substituent affects regioselectivity in the reductive elimination step. Palladium-catalyzed annulation of silacyclo- butanes and 2-iodobiarenes was selected as model reaction, and detailed reaction mechanism is illustrated. The results shows that the reaction undergoes Pd—I bond oxidative addition, concerted metalation deprotonation (CMD), Pd—Si bond oxidative addition, and reductive elimination process to synthesize eight-membered silacycles, and C—Si bond reductive elimination is the rate determining step. Study of electronic effect in Pd(IV) reductive elimination transition state shows that when asymmetrically substituted 2-iodobyphenyl is used as substrate, electron density of aromatic ring is the main factor to control regioselectivity. Groups with higher electron density have higher priority for reductive elimination, and this is in accordance with electron flow in this elementary reaction.

| [1] | (a) Liu P.; Montgomery J.; Houk K. N. J. Am. Chem. Soc. 2011, 133, 6956. |
| [1] | (b) Bronner S. M.; Mackey J. L.; Houk K. N.; Garg N. K. J. Am. Chem. Soc. 2012, 134, 13966. |
| [1] | (c) Wright J. S.; Scott P. J. H.; Steel P, G. Angew. Chem. Int. Ed. 2021, 60, 2796. |
| [2] | (a) Kakiuchi F.; Kochi T.; Mutsutani H.; Kobyashi N.; Urano S.; Sato M.; Nishiyama S.; Tanabe T. J. Am. Chem. Soc. 2009, 131, 11310. |
| [2] | (b) Liu P.; Sirois L. E.; Cheong P. H. Y.; Yu Z. X.; Hartung I. V.; Rieck H.; Wender P. A.; Houk K. N. J. Am. Chem. Soc. 2010, 132, 10127. |
| [2] | (c) Medina J. M.; Mackey J. L.; Garg N. K.; Houk K. N. J. Am. Chem. Soc. 2014, 136, 15798. |
| [2] | (d) Tomioka K.; Shioya Y.; Nagaoka Y.; Yamada K. J. Org. Chem. 2001, 66, 7051. |
| [2] | (e) Baral R. N.; Thomas S. W. J. Org. Chem. 2015, 80, 11086. |
| [2] | (f) Moore L. P.; Hagedorn Z. J.; Barnes M. E.; Dye M. L. N.; Whitt L. M.; Wilger D. J. Organometallics 2023, 42, 357. |
| [2] | (g) Karmakar R.; Yun S. Y.; Wang K. P.; Lee D. Org. Lett. 2014, 16, 6. |
| [3] | Liu S. H.; Zhang T.; Zhu L.; Liu F. R. Bai R. P.; Lan Y. Org. Lett. 2020, 22, 2124. |
| [4] | Huo J. F.; Zhong K. B.; Xue Y. Z.; Lyu M. M.; Ping Y. F.; Ouyang W. B.; Liu Z. X.; Lan Y.; Wang J. B. Chem.-Eur. J. 2022, 28, e202200191. |
| [5] | (a) Lai W.; Zhong K. B.; Liu S.; Liu S. H.; Chen H. H.; Ni H.; Zeng Z. Zhao Z.; Lan Y.; Bai R. P. J. Phys. Chem. Lett. 2022, 13, 7694. |
| [5] | (b) Chen S. W.; He X. Q.; Jin C. Y.; Zhang W. L.; Yang Y. X.; Liu S. S. Lan Y.; Houk K. N.; Shen X. Angew. Chem. Int. Ed. 2022, 61, e202213431. |
| [5] | (c) Hirano K. Yorimitsu H.; Oshima K. Org. Lett. 2006, 8, 483. |
| [6] | (a) Zhang Y.; Ma J. W.; Chen J.; Meng L. P. Liang Y.; Zhu S. L. Chem. 2021, 7, 3171. |
| [6] | (b) Zhang Y. L.; He J.; Song P. H. Wang Y.; Zhu S. L. CCS Chem. 2020, 2, 2259. |
| [6] | (c) He Y. L.; Han B.; Zhu S. L. Organometallics 2021, 40, 2253. |
| [7] | (a) Zhao W. T.; Gao F.; Zhao D. Angew. Chem., Int. Ed. 2018, 57, 6329. |
| [7] | (b) Ishida N.; Ikemoto W.; Murakami M. J. Am. Chem. Soc. 2014, 136, 5912. |
| [7] | (c) Okumura S.; Sun F.; Ishida N.; Murakami M. J. Am. Chem. Soc. 2017, 139, 12414. |
| [8] | Zhu M. H.; Zhang X. W.; Usman M.; Cong H. J.; Liu W. B. ACS Catal. 2021, 11, 5703. |
| [9] | Zhang J.; Zhang T.; Liu T.; Lan Y. Dalton Trans. 2021, 50, 7656. |
| [10] | (a) Chen H.; Peng J.; Pang Q. J.; Du H. M.; Huang L. Y.; Lu Gao Lan, Y.; Yang C.; Song Z. L. Angew. Chem. Int. Ed. 2022, e202212889. |
| [10] | (b) Li L. J.; Zhang Y. B.; Gao L.; Song Z. L. Tetrahedron Lett. 2015, 56, 1466. |
| [11] | Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A. V.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian H. P.; Ortiz J. V.; Izmaylov A. F.; Sonnenberg J. L.; Williams-Young D.; Ding F.; Lipparini F.; Egidi F.; Goings J.; Peng B.; Petrone A.; Henderson T.; Ranasinghe D.; Zakrzewski V. G.; Gao J.; Rega N.; Zheng G.; Liang W.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery J. A., Jr.; Peralta J. E.; Ogliaro F.; Bearpark M. J.; Heyd J. J.; Brothers E. N.; Kudin K. N.; Staroverov V. N.; Keith T. A.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A. P.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Millam J. M.; Klene M.; Adamo C.; Cammi R.; Ochterski J. W.; Martin R. L.; Morokuma K.; Farkas O.; Foresman J. B.; Fox D. J. Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016. |
| [12] | (a) Becke A. D. J. Chem. Phys. 1993, 98, 5648. |
| [12] | (b) Stephens P. J.; Devlin F. J.; Chabalowski C. F.; Frisch M. J. J. Phys. Chem. 1994, 98, 11623. |
| [12] | (c) Lee C.; Yang W.; Parr R. G. Phys. Rev. B: Condens. Matter Mater. Phys. 1988, 37, 785. |
| [13] | Cancès E.; Mennucci B.; Tomasi J. J. Chem. Phys. 1997, 107, 3032. |
| [14] | (a) Zhao Y.; Truhlar D. Theor. Chem. Acc. 2008, 120, 215. |
| [14] | (b) Qi X.; Zhang H.; Shao A.; Zhu L.; Xu T.; Gao M.; Liu C.; Lan Y. ACS Catal. 2015, 5, 6640. |
| [14] | (c) Zhao Y.; Truhlar D. G. J. Chem. Phys. 2006, 125, 194101. |
| [15] | Weigend F.; Ahlrichs R. Phys. Chem. Chem. Phys. 2005, 7, 3297. |
| [16] | (a) Li Y.; Liu S.; Qi Z.; Qi X.; Li X.; Lan Y. Chem. Eur. J. 2015, 21, 10131. |
| [16] | (b) Qi X.; Li Y.; Zhang G.; Li Y.; Lei A.; Liu C.; Lan Y. Dalton Trans. 2015, 44, 11165. |
| [16] | (c) Marenich A. V.; Cramer C. J.; Truhlar D. G. J. Phys. Chem. B 2009, 113, 6378. |
| [16] | (d) Marenich A. V.; Cramer C. J.; Truhlar D. G. J. Phys. Chem. B 2009, 113, 4538. |
| [16] | (e) Yu Z.; Qi X.; Li Y.; Liu S.; Lan Y. Org. Chem. Front. 2016, 3, 209. |
| [17] | Legault C. Y. CYLview 1.0b, Université de Sherbrooke, Canada, 2009, http://www.cylview.org. |
/
| 〈 |
|
〉 |