

电化学促使α-重氮酯的磷酸化构筑亚膦酸腙
收稿日期: 2023-09-21
修回日期: 2023-11-13
网络出版日期: 2023-11-23
基金资助
国家自然科学基金(21902083); 山东省自然科学基金(ZR2020QB130); 曲阜师范大学人才启动基金(6132); 曲阜师范大学人才启动基金(6125)
Electrochemical Enabled Phosphorylation of α-Diazoester to Access Phosphinic Hydrazone
Received date: 2023-09-21
Revised date: 2023-11-13
Online published: 2023-11-23
Supported by
National Natural Science Foundation of China(21902083); Natural Science Foundation of Shandong Province(ZR2020QB130); Talent Program Foundation of Qufu Normal University(6132); Talent Program Foundation of Qufu Normal University(6125)
孙雪 , 颜廷涛 , 闫克鲁 , 杨建静 , 文江伟 . 电化学促使α-重氮酯的磷酸化构筑亚膦酸腙[J]. 有机化学, 2024 , 44(3) : 1013 -1020 . DOI: 10.6023/cjoc202309022
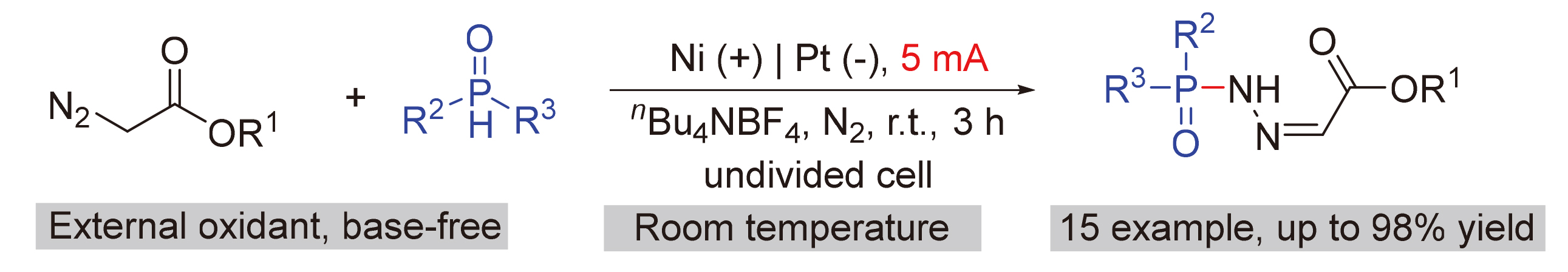
The phosphonic hydrazone represents a rare molecular fragment with promising applications in the fields of pharmaceuticals, functional materials, ligands, and synthetic intermediates. A novel electrochemical approach is presented for the generation of Ni2+ ions from sacrificial anode nickel, facilitating the phosphorylation of α-diazoester to construct phosphite hydrazone. The reaction was performed in an undivided cell in absence of precious metal catalyst and chemical redox reagent, exhibiting good substrate applicability. The mechanistic investigation has confirmed the predominant role of paired electrolysis in mediating the catalytic mechanism.
| [1] |
(a) Biçer E. Phosphorus, Sulfur Silicon Relat. Elem. 2021, 196, 791.
|
| [1] |
(b) Cai B.-G.; Xuan J.; Xiao W.-J. Sci. Bull. 2019, 64, 337.
|
| [2] |
(a) Audrieth L. F.; Gher R. J.; Smith W. C. J. Org. Chem. 1955, 20, 1288.
|
| [2] |
(b) Ephraim F.; Sackheim M. Ber. Dtsch. Chem. Ges. 1911, 44, 3416.
|
| [2] |
(c) Tolkmith H.; Britton E. J. Org. Chem. 1959, 24, 705.
|
| [3] |
(a) Boaz N. W.; Mackenzie E. B.; Debenham S. D.; Large S. E.; Ponasik J. A. J. Org. Chem. 2005, 70, 1872.
|
| [3] |
(b) Wang D.-Y.; Huang J.-D.; Hu X.-P.; Deng J.; Yu S.-B.; Duan Z.-C.; Zheng Z. J. Org. Chem. 2008, 73, 2011.
|
| [3] |
(c) Gross T.; Chou S.; Dyke A.; Dominguez B.; Groarke M.; Medlock J.; Ouellette M.; Reddy J. P.; Seger A.; Zook S.; Zanotti-Gerosa A. Tetrahedron Lett. 2012, 53, 1025.
|
| [3] |
(d) You C.; Li S.; Li X.; Lv H.; Zhang X. ACS Catal. 2019, 9, 8529.
|
| [3] |
(e) Schlatzer T.; Breinbauer R. Adv. Synth. Catal. 2021, 363, 668.
|
| [4] |
Select recent representative examples: a Wang, L.; Wu, Y.; Liu, Y.; Yang, H.; Liu, X.; Wang, J.; Li, X.; Jiang, J. Org. Lett. 2017, 19, 782.
|
| [4] |
(b) Ciszewski ?. W.; Durka J.; Gryko D. Org. Lett. 2019, 21, 7028.
|
| [4] |
(c) Su Y.-L.; Liu G.-X.; Liu J.-W.; Tram L.; Qiu H.; Doyle M. P. J. Am. Chem. Soc. 2020, 142, 13846.
|
| [4] |
(d) He F.; Koenigs R. M. Org. Lett. 2021, 23, 5831.
|
| [4] |
(e) Shou J.-Y.; Xu X.-H.; Qing F.-L. Angew. Chem., Int. Ed. 2021, 60, 15271.
|
| [4] |
(f) Stivanin M. L.; Duarte M.; Le?o L. P. M. O.; Saito F. A.; Jurberg I. D. J. Org. Chem. 2021, 86, 17528.
|
| [4] |
(g) Wang F.; Nishimoto Y.; Yasuda M. J. Am. Chem. Soc. 2021, 143, 20616.
|
| [4] |
(h) Chen R.; Ma G.; Li Y.; Zhang J.; Xia R.; Wang K.-K.; Liu L. J. Org. Chem. 2022, 87, 10990.
|
| [4] |
(i) Lv Y.; Liu R.; Ding H.; Wei W.; Zhao X.; He L. Org. Chem. Front. 2022, 9, 3486.
|
| [4] |
(j) Shou J.-Y.; Qing F.-L. Angew. Chem., Int. Ed. 2022, 61, e202208860.
|
| [4] |
(k) Sun Y.-T.; Rao X.; Xu W.; Xu M.-H. Org. Chem. Front. 2022, 9, 3467.
|
| [5] |
(a) Tolkmith H. J. Am. Chem. Soc. 1962, 84, 2097.
|
| [5] |
(b) Cates L. A.; Cho Y. M.; Smith L. K.; Williams L.; Lemke T. L. J. Med. Chem. 1976, 19, 1133.
|
| [5] |
(c) Bagrov F.; Matveeva T.; Petrukhin V. Chem. Technol. Fuels Oils 1997, 33, 41.
|
| [6] |
Audrieth L.; Gher JR R.; Smith W. C. J. Org. Chem. 1955, 20, 1288.
|
| [7] |
(a) Jiang H.; Jin H.; Abdukader A.; Lin A.; Cheng Y.; Zhu C. Org. Biomol. Chem. 2013, 11, 3612.
|
| [7] |
(b) Gu X.; Xie P.; Jiang J.; Wu Y.; Wang L. J. Chem. Res. 2018, 42, 63.
|
| [8] |
Balázs L. B.; Huang Y.; Khalikuzzaman J. B.; Li Y.; Pullarkat S. A.; Leung P.-H. J. Org. Chem. 2020, 85, 14763.
|
| [9] |
Recent representative reviews: (a) Yan, M.; Kawamata, Y.; Baran, P. S. Chem. Rev. 2017, 117, 13230.
|
| [9] |
(b) Jiang Y.; Xu K.; Zeng C. Chem. Rev. 2018, 118, 4485.
|
| [9] |
(c) Liu Y.; Yi H.; Lei A. Chin. J. Chem. 2018, 36, 692.
|
| [9] |
(d) Wang H.; Gao X.; Lv Z.; Abdelilah T.; Lei A. Chem. Rev. 2019, 119, 6769.
|
| [9] |
(e) Xiong P.; Xu H. C. Acc. Chem. Res. 2019, 52, 3339.
|
| [9] |
(f) Jiao K. J.; Xing Y. K.; Yang Q. L.; Qiu H.; Mei T. S. Acc. Chem. Res. 2020, 53, 300.
|
| [9] |
(g) Yamamoto K.; Kuriyama M.; Onomura O. Acc. Chem. Res. 2020, 53, 105.
|
| [9] |
(h) Novaes L. F. T.; Liu J.; Shen Y.; Lu L.; Meinhardt J. M.; Lin S. Chem. Soc. Rev. 2021, 50, 7941.
|
| [9] |
(i) Yang J.; Qin H.; Yan K.; Cheng X.; Wen J. Adv. Synth. Catal. 2021, 363, 5407.
|
| [9] |
(j) Yang J.; Ma J.; Yan K.; Tian L.; Li B.; Wen J. Adv. Synth. Catal. 2022, 364, 845.
|
| [9] |
(k) Zhou H.-Y.; Tang H.-T.; He W.-M. Chin. J. Catal. 2023, 46, 4.
|
| [9] |
(l) Li Q.-Y.; Swaroop T. R.; Hou C.; Wang Z.-Q.; Pan Y. M.; Tang H.-T. Adv. Synth. Catal. 2019, 361, 1761.
|
| [9] |
(m) He M.; Cheng S.; Pan Y.; Tang H. T.; Pan Y. M. Chin. J. Org. Chem. 2021, 41, 2354. (in Chinese)
|
| [9] |
( 何慕雪, 程诗砚, 潘永周, 唐海涛, 潘英明, 有机化学, 2021, 41, 2354.)
|
| [9] |
(n) Zheng Y.; Qian S.; Xu P.; Zheng B.; Huang S. L. Chin. J.Org. Chem. 2022, 42, 4275. (in Chinese)
|
| [9] |
( 郑煜, 钱沈城, 徐鹏程, 郑斌南, 黄申林, 有机化学, 2022, 42, 4275.)
|
| [9] |
(o) Xu H.; Meng X.; Zheng Y.; Luo J.; Huang S. L. Chin. J. Org. Chem. 2021, 41, 4696. (in Chinese)
|
| [9] |
( 徐鹤华, 孟祥太, 郑煜, 罗金岳, 黄申林, 有机化学, 2021, 41, 4696.)
|
| [10] |
Yuan Y.; Liu X.; Hu J.; Wang P.; Wang S.; Alhumade H.; Lei A. Chem. Sci. 2022, 13, 3002.
|
| [11] |
(a) Sun X.; Yang J.; Yan K.; Zhuang X.; Yu J.; Song X.; Zhang F.; Li B.; Wen J. Chem. Commun. 2022, 58, 8238.
|
| [11] |
(b) Yang J.; Sun X.; Yan K.; Sun H.; Sun S.; Jia X.; Zhang F.; Wen J. Adv. Synth. Catal. 2022, 364, 2735.
|
/
| 〈 |
|
〉 |