

电化学芳烃C(sp2)—H胺化反应的研究进展
收稿日期: 2023-10-31
修回日期: 2023-12-13
网络出版日期: 2024-01-05
基金资助
国家重点研发计划(2022YFA1503200); 国家自然科学基金(22371149); 国家自然科学基金(22188101); 中央高校基本科研业务费专项资金(63223015); 南开大学有机新物质创造前沿科学中心(63181206)
Recent Advance in Electrochemical C(sp2)—H Amination of Arenes
Received date: 2023-10-31
Revised date: 2023-12-13
Online published: 2024-01-05
Supported by
National Key Research and Development Program of China(2022YFA1503200); National Natural Science Foundation of China(22371149); National Natural Science Foundation of China(22188101); Fundamental Research Funds for the Central Universities(63223015); Frontiers Science Center for New Organic Matter, Nankai University(63181206)
朱子乐 , 李鹏飞 , 仇友爱 . 电化学芳烃C(sp2)—H胺化反应的研究进展[J]. 有机化学, 2024 , 44(3) : 871 -891 . DOI: 10.6023/cjoc202310033
Aniline and its derivatives are widely used and consumed in human life and industrial production, which inspires the direct aromatic C(sp2)—N construction from the corresponding C(sp2)—H bond. In recent years, as a controllable, sustainable, ambient, and highly scalable methodology, organic electrochemistry has received greater attention and also combined aromatic C(sp2)—N amination, presenting novel reactions. In this review, the common mechanism manifolds of electrochemical aromatic C(sp2)—H amination reactions are summarized, and the reactions examples are classified according to the type of amine sources. The prospects and challenges in this field are provided.
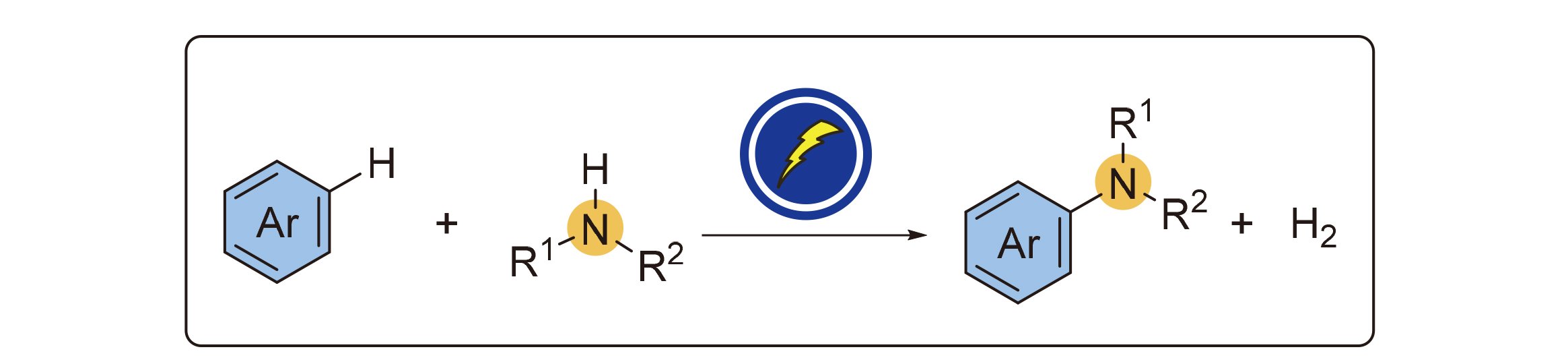
Key words: electrochemistry; amination; arene; aniline; C—H amination
| [1] | Amini B.; Lowenkron S. In Kirk-Othmer Encyclopedia of Chemical Technology, Ed.: Kirk-Othmer, Wiley, New York, 2003. |
| [2] | Rappoport Z. The Chemistry of Anilines, 1st ed., Wiley, Chichester, 2007. |
| [3] | Kahl T.; Schr?der K.-W.; Lawrence F. R.; Marshall W. J.; H?ke H.; J?ckh R.In Ullmann's Encyclopedia of Industrial Chemistry, Eds.: Elvers, B.; Bellussi, G., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011, p. 465-477. |
| [4] | Anjalin M.; Kanagathara N.; Suganthi A. R. B. Mater. Today Proc. 2020, 33, 4751. |
| [5] | Shuja M. H.; Shuja S. H.; Shakil F.; Ahmed I. Ann. Med. Surg. 2023, 85, 1346. |
| [6] | Niu W.; Li L.; Liu X.; Wang N.; Liu J.; Zhou W.; Tang Z.; Chen S. J. Am. Chem. Soc. 2015, 137, 5555. |
| [7] | Vervoort J.; De Jager P. A.; Steenbergen J.; Rietjens I. M. C. M. Xenobiotica 1990, 20, 657. |
| [8] | Duckett C. J.; Lindon J. C.; Walker H.; Abou-Shakra F.; Wilson I. D; Nicholson J. K. Xenobiotica 2006, 36, 59. |
| [9] | MacKetta J. J. In Encyclopedia of Chemical Processing and Design, Dekker, New York, 1990. |
| [10] | Béchamp Reduction, In Comprehensive Organic Name Reactions and Reagents, Wiley, Hoboken, NJ, 2010, pp. 284-287. |
| [11] | Porter H. K. In Organic Reactions, Ed.: Denmark, S. E., Wiley, 2011, pp. 455-481, doi: 10.1002/0471264180.or074.03. |
| [12] | Driessen R. T.; Kamphuis P.; Mathijssen L.; Zhang R.; Van Der Ham, L. G. J.; Van Den Berg, H.; Zeeuw, A. J. Chem. Eng. Technol. 2017, 40, 838. |
| [13] | Moreno S. N.; Docampo R. Environ. Health Perspect. 1985, 64, 199. |
| [14] | Goldberg I. Ber. Dtsch. Chem. Ges. 1906, 39, 1691. |
| [15] | Allen C. F. H.; McKee G. H. W. Org. Synth. 1939, 19, 6. |
| [16] | Paul F.; Patt J.; Hartwig J. F. J. Am. Chem. Soc. 1994, 116, 5969. |
| [17] | Guram A. S.; Buchwald S. L. J. Am. Chem. Soc. 1994, 116, 7901. |
| [18] | Chan D. M. T.; Monaco K. L.; Wang R.-P.; Winters M. P. Tetrahedron Lett. 1998, 39, 2933. |
| [19] | Lam P. Y. S.; Clark C. G.; Saubern S.; Adams J.; Winters M. P.; Chan D. M. T.; Combs A. Tetrahedron Lett. 1998, 39, 2941. |
| [20] | Burns N. Z.; Baran P. S.; Hoffmann R. W. Angew. Chem., Int. Ed. 2009, 48, 2854. |
| [21] | Louillat M.-L.; Patureau F. W. Chem. Soc. Rev. 2014, 43, 901. |
| [22] | Park Y.; Kim Y.; Chang S. Chem. Rev. 2017, 117, 9247. |
| [23] | Yang Y.; Zhang D.; Vessally E. Top. Curr. Chem. 2020, 378, 37. |
| [24] | Beletskaya I. P.; Averin A. D. Russ. Chem. Rev. 2021, 90, 1359. |
| [25] | Feng Y.-L.; Shi B.-F. Chin. J. Org. Chem. 2021, 41, 3753. (in Chinese) |
| [25] | ( 冯亚岚, 史炳锋, 有机化学, 2021, 41, 3753.) |
| [26] | Ravindar L.; Hasbullah S. A.; Hassan N. I.; Qin H. Eur. J. Org. Chem. 2022, 31, e202200596. |
| [27] | Krishna Rao M. V.; Kareem S.; Vali S. R.; Subba Reddy B. V. Org. Biomol. Chem. 2023, 21, 8426. |
| [28] | Boursalian G. B.; Ngai M. Y.; Hojczyk K. N.; Ritter T. J. Am. Chem. Soc. 2013, 135, 13278. |
| [29] | Roy S.; Panja S.; Sahoo S. R.; Chatterjee S.; Maiti D. Chem. Soc. Rev. 2023, 52, 2391. |
| [30] | Mu?iz K. Acc. Chem. Res. 2018, 51, 1507. |
| [31] | K?rk?s M. D. Chem. Soc. Rev. 2018, 47, 5786. |
| [32] | Liu C.; Liu J.; Li W.; Lu H.; Zhang Y. Org. Chem. Front. 2023, 10, 5309. |
| [33] | Zhang H.; Lei A. Synthesis 2019, 51, 83. |
| [34] | Meng Z.; Feng C.; Xu K. Chin. J. Org. Chem. 2021, 41, 2535. (in Chinese) |
| [34] | ( 蒙泽银, 冯承涛, 徐坤, 有机化学, 2021, 41, 2535.) |
| [35] | Chen N.; Xu H. Green Synth. Catal. 2021, 2, 165. |
| [36] | Wang H.; Gao X.; Lv Z.; Abdelilah T.; Lei A. Chem. Rev. 2019, 119, 6769. |
| [37] | Lu L.; Shi R.; Lei A. Trends Chem. 2022, 4, 179. |
| [38] | Cavedon C.; Seeberger P. H.; Pieber B. Eur. J. Org. Chem. 2020, 2020, 1379. |
| [39] | Singh S.; Roy V. J.; Dagar N.; Sen P. P.; Roy S. R. Adv. Synth. Catal. 2021, 363, 937. |
| [40] | Kwon K.; Simons R. T.; Nandakumar M.; Roizen J. L. Chem. Rev. 2022, 122, 2353. |
| [41] | Holmberg-Douglas N.; Nicewicz D. A. Chem. Rev. 2022, 122, 1925. |
| [42] | Chan C.; Chow Y.; Yu W. Synthesis 2020, 52, 2899. |
| [43] | Frontana-Uribe B. A.; Little R. D.; Ibanez J. G.; Palma A.; Vasquez-Medrano R. Green Chem. 2010, 12, 2099. |
| [44] | Luca O. R.; Gustafson J. L.; Maddox S. M.; Fenwick A. Q.; Smith D. C. Org. Chem. Front. 2015, 2, 823. |
| [45] | Yan M.; Kawamata Y.; Baran P. S. Chem. Rev. 2017, 117, 13230. |
| [46] | Wiebe A.; Gieshoff T.; M?hle S.; Rodrigo E.; Zirbes M.; Waldvogel S. R. Angew. Chem., Int. Ed. 2018, 57, 5594. |
| [47] | Shida N.; Zhou Y.; Inagi S. Acc. Chem. Res. 2019, 52, 2598. |
| [48] | Kingston C.; Palkowitz M. D.; Takahira Y.; Vantourout J. C.; Peters B. K.; Kawamata Y.; Baran P. S. Acc. Chem. Res. 2020, 53, 72. |
| [49] | Novaes L. F. T.; Liu J.; Shen Y.; Lu L.; Meinhardt J. M.; Lin S. Chem. Soc. Rev. 2021, 50, 7941. |
| [50] | Feng T.; Wang S.; Qiu Y. Synlett 2022, 33, 1582. |
| [51] | Ogibin Y. N.; Elinson M. N.; Nikishin G. I. Russ. Chem. Rev. 2009, 78, 89. |
| [52] | Francke R.; Little R. D. Chem. Soc. Rev. 2014, 43, 2492. |
| [53] | Tay N. E. S.; Lehnherr D.; Rovis T. Chem. Rev. 2022, 122, 2487. |
| [54] | Mruthunjaya A. K. V.; Torriero A. A. J. Molecules 2023, 28, 471. |
| [55] | Roth H.; Romero N.; Nicewicz D. Synlett 2015, 27, 714. |
| [56] | Wawzonek S.; McIntyre T. W. J. Electrochem. Soc. 1967, 114, 1025. |
| [57] | Dvo?ák V.; Němec I.; Zyka J. Microchem. J. 1967, 12, 99. |
| [58] | Paduszek B.; Kalinowski M. K. Electrochim. Acta 1983, 28, 639. |
| [59] | Loveland J. W.; Dimeler G. R. Anal. Chem. 1961, 33, 1196. |
| [60] | O’Donnell, J. F.; Mann, C. K. J. Electroanal. Chem. Interfacial Electrochem. 1967, 13, 157. |
| [61] | Merkel P. B.; Luo P.; Dinnocenzo J. P.; Farid S. J. Org. Chem. 2009, 74, 5163. |
| [62] | Kita Y.; Tohma H.; Hatanaka K.; Takada T.; Fujita S.; Mitoh S.; Sakurai H.; Oka S. J. Am. Chem. Soc. 1994, 116, 3684. |
| [63] | Ischay M. A.; Yoon T. P. Eur. J. Org. Chem. 2012, 2012, 3359. |
| [64] | Yi H.; Zhang G.; Wang H.; Huang Z.; Wang J.; Singh A. K.; Lei A. Chem. Rev. 2017, 117, 9016. |
| [65] | Cui H.-L. Org. Biomol. Chem. 2020, 18, 2975. |
| [66] | Pistritto V. A.; Liu S.; Nicewicz D. A. J. Am. Chem. Soc. 2022, 144, 15118. |
| [67] | Michejda C. J. W.; Hoss P. J. Am. Chem. Soc. 1970, 92, 6298. |
| [68] | Danen W. C.; Neugebauer F. A. Angew. Chem. Int. Ed. Engl. 1975, 14, 783. |
| [69] | Chow Y. L.; Danen W. C.; Nelsen S. F.; Rosenblatt D. H. Chem. Rev. 1978, 78, 243. |
| [70] | Zard S. Z. Chem. Soc. Rev. 2008, 37, 1603. |
| [71] | Xiong T.; Zhang Q. Chem. Soc. Rev. 2016, 45, 3069. |
| [72] | Boursalian G. B.; Ham W. S.; Mazzotti A. R.; Ritter T. Nat. Chem. 2016, 8, 810. |
| [73] | Pratley C.; Fenner S.; Murphy J. A. Chem. Rev. 2022, 122, 8181. |
| [74] | Gao W.; Li W.; Zeng C.; Tian H.; Hu L.; Little R. J. Org. Chem. 2014, 79, 9613. |
| [75] | Qiu Y.; Struwe J.; Meyer T. H.; Oliveira J. C. A.; Ackermann L. Chem.-Eur. J. 2018, 24, 12784. |
| [76] | Gao X.; Wang P.; Zeng L.; Tang S.; Lei A. J. Am. Chem. Soc. 2018, 140, 4195. |
| [77] | Sauermann N.; Mei R.; Ackermann L. Angew. Chem., Int. Ed. 2018, 57, 5090. |
| [78] | Zhang S.; Samanta R. C.; Sauermann N.; Ackermann L. Chem.- Eur. J. 2018, 24, 19166. |
| [79] | Kathiravan S.; Suriyanarayanan S.; Nicholls I. A. Org. Lett. 2019, 21, 1968. |
| [80] | Yang Q.; Wang X.; Lu J.; Zhang L.; Fang P.; Mei T. J. Am. Chem. Soc. 2018, 140, 11487. |
| [81] | Tang S.; Wang S.; Liu Y.; Cong H.; Lei A. Angew. Chem. Int. Ed. 2018, 57, 4737. |
| [82] | Liu K.; Tang S.; Wu T.; Wang S.; Zou M.; Cong H.; Lei A. Nat. Commun. 2019, 10, 639. |
| [83] | Wu Y.; Jiang S.; Song R.; Li J. Chem. Commun. 2019, 55, 4371. |
| [84] | Chen S.; Li Y.; Xiang S.; Li S.; Tan B. Chem. Commun. 2021, 57, 8512. |
| [85] | Feng C.; Liu X.; She Y.; Shen Z.; Li M. Chin. Chem. Lett. 2023, 34, 107935. |
| [86] | Wang Z.; Cheng Q.; Peng R.; Yan P.; Zeng R.; Tian W.; Pan B.; Gu J.; Li Y.; Ouyang Q. J. Org. Chem. 2022, 87, 4742. |
| [87] | Zincke Th.; Heuser G.; M?ller W. Justus Liebigs Ann. Chem. 1904, 333, 296. |
| [88] | Ritter J. J.; Kalish J. J. Am. Chem. Soc. 1948, 70, 4048. |
| [89] | Lund H.; Tegnér C.; Takman B. Acta Chem. Scand. 1957, 11, 1323. |
| [90] | Shine H. J.; Silber J. J.; Bussey R. J.; Okuyama T. J. Org. Chem. 1972, 37, 2691. |
| [91] | Blackburn G. M.; Will J. P. J. Chem. Soc., Chem. Commun. 1974, 67. |
| [92] | Ruhlmann L.; Schulz A.; Giraudeau A.; Messerschmidt C.; Fuhrhop J. H. J. Am. Chem. Soc. 1999, 121, 6664. |
| [93] | Li Y.; Kamata K.; Kawai T.; Abe J.; Iyoda T. J. Chem. Soc., Perkin 1 2002, 1135. |
| [94] | Li Y.; Asaoka S.; Yamagishi T.; Iyoda T. Electrochemistry 2004, 72, 171. |
| [95] | Morofuji T.; Shimizu A.; Yoshida J. Angew. Chem., Int. Ed. 2012, 51, 7259. |
| [96] | Morofuji T.; Shimizu A.; Yoshida J. J. Am. Chem. Soc. 2013, 135, 5000. |
| [97] | Herold S.; M?hle S.; Zirbes M.; Richter F.; Nefzger H.; Waldvogel S. R. Eur. J. Org. Chem. 2016, 2016, 1274. |
| [98] | M?hle S.; Herold S.; Richter F.; Nefzger H.; Waldvogel S. R. ChemElectroChem 2017, 4, 2196. |
| [99] | Wesenberg L. J.; Herold S.; Shimizu A.; Yoshida J.; Waldvogel S. R. Chem.-Eur. J. 2017, 23, 12096. |
| [100] | Strekalova S.; Kononov A.; Rizvanov I.; Budnikova Y. RSC Adv. 2021, 11, 37540. |
| [101] | Taily I. M.; Saha D.; Banerjee P. Org. Lett. 2022, 24, 2310. |
| [102] | Fu Y.; Zhang L.; Sun M.; Cao L.; Yang L.; Cheng R.; Ma Y.; Ye J. Eur. J. Org. Chem. 2023, 26, e202300553. |
| [103] | Morofuji T.; Shimizu A.; Yoshida J. J. Am. Chem. Soc. 2014, 136, 4496. |
| [104] | Morofuji T.; Shimizu A.; Yoshida J. J. Am. Chem. Soc. 2015, 137, 9816. |
| [105] | De Robillard G.; Makni O.; Cattey H.; Andrieu J.; Devillers C. H. Green Chem. 2015, 17, 4669. |
| [106] | Yu Y.; Yuan Y.; Liu H.; He M.; Yang M.; Liu P.; Yu B.; Dong X.; Lei A. Chem. Commun. 2019, 55, 1809. |
| [107] | Wang J.-H.; Lei T.; Nan X.-L.; Wu H.-L.; Li X.-B.; Chen B.; Tung C.-H.; Wu L.-Z. Org. Lett. 2019, 21, 5581. |
| [108] | Sun M.; Zhou Y.; Li L.; Wang L.; Ma Y.; Li P. Org. Chem. Front. 2021, 8, 754. |
| [109] | Buglioni L.; Besla? M.; No?l T. J. Org. Chem. 2021, 86, 16195. |
| [110] | Zhou N.; Zhao J.; Sun C.; Lai Y.; Ruan Z.; Feng P. J. Org. Chem. 2021, 86, 16059. |
| [111] | Xu H.-C.; Campbell J. M.; Moeller K. D. J. Org. Chem. 2014, 79, 379. |
| [112] | Hu X.; Zhang G.; Nie L.; Kong T.; Lei A. Nat. Commun. 2019, 10, 5467. |
| [113] | Zhang Y.; Lin Z.; Ackermann L. Chem.-Eur. J. 2021, 27, 242. |
| [114] | Peng X.; Zhao J.; Ma G.; Wu Y.; Hu S.; Ruan Z.; Feng P. Green Chem. 2021, 23, 8853. |
| [115] | Puthanveedu M.; Khamraev V.; Brieger L.: Strohmann C.; Antonchick A. P. Chem.-Eur. J. 2021, 27, 8008. |
| [116] | Zhang Y.-Z.; Mo Z.-Y.; Wang H.-S.; Wen X.-A.; Tang H.-T.; Pan Y.-M. Green Chem. 2019, 21, 3807. |
| [117] | Luo M.-J.; Ouyang X.-H.; Zhu Y.-P.; Li Y.; Li J.-H. Green Chem. 2021, 23, 9024. |
| [118] | Wang H.; Zheng Y.; Xu H.; Zou J.; Jin C. Front. Chem. 2022, 10, 950635. |
| [119] | Morofuji T.; Shimizu A.; Yoshida J. Chem.-Eur. J. 2015, 21, 3211. |
| [120] | Ohno Y.; Ando S.; Furusho D.; Hifumi R.; Nagata Y.; Tomita I.; Inagi S. Org. Lett. 2023, 25, 3951. |
| [121] | Zhao H.; Liu Z.; Song J.; Xu H. Angew. Chem., Int. Ed. 2017, 56, 12732. |
| [122] | Zhao H.; Xu P.; Song J.; Xu H. Angew. Chem., Int. Ed. 2018, 57, 15153. |
| [123] | Zhang S.; Li L.; Xue M.; Zhang R.; Xu K.; Zeng C. Org. Lett. 2018, 20, 3443. |
| [124] | Zhang P.; Li B.; Niu L.; Wang L.; Zhang G.; Jia X.; Zhang G.; Liu S.; Ma L.; Gao W.; Qin D.; Chen J. Adv. Synth. Catal. 2020, 362, 2342. |
| [125] | Wang Q.; Zhang X.; Wang P.; Gao X.; Zhang H.; Lei A. Chin. J. Chem. 2021, 39, 143. |
| [126] | Zhang H.; Ye Z.; Chen N.; Chen Z.; Zhang F. Green Chem. 2022, 24, 1463. |
| [127] | Wan H.; Li D.; Xia H.; Yang L.; Alhumade H.; Yi H.; Lei A. Chem. Commun. 2022, 58, 665. |
| [128] | Zhao H.; Hou Z.; Liu Z.; Zhou Z.; Song J.; Xu H. Angew. Chem., Int. Ed. 2017, 56, 587. |
| [129] | Zhao H.; Zhuang J.; Xu H. ChemSusChem 2021, 14, 1692. |
| [130] | Duan Z.; Zhang L.; Zhang W.; Lu L.; Zeng L.; Shi R.; Lei A. ACS Catal. 2020, 10, 3828. |
| [131] | Yang G.; Wang Y.; Qiu Y. Chin. J. Org. Chem. 2021, 41, 3935. (in Chinese) |
| [131] | ( 杨光, 王衍伟, 仇友爱, 有机化学, 2021, 41, 3935.) |
| [132] | Li P.; Zhang T.; Mushtaq M. A.; Wu S.; Xiang X.; Yan D. Chem. Rec. 2021, 21, 841. |
| [133] | Huang H.; Steiniger K. A.; Lambert T. H. J. Am. Chem. Soc. 2022, 144, 12567. |
| [134] | Qian L.; Shi M. Chem. Commun. 2023, 59, 3487. |
| [135] | Decker F.; Cattarin S. In Encyclopedia of Electrochemical Power Sources, Elsevier, Amsterdam, 2009, pp. 1-9. |
| [136] | Huang H.; Strater Z. M.; Rauch M.; Shee J.; Sisto T. J.; Nuckolls C.; Lambert T. H. Angew. Chem., Int. Ed. 2019, 58, 13318. |
| [137] | Hou Z.; Xu H. ChemElectroChem 2021, 8, 1571. |
| [138] | Wu S.; ?urauskas J.; Domański M.; Hitzfeld P. S.; Butera V.; Scott D. J.; Rehbein J.; Kumar A.; Thyrhaug E.; Hauer J.; Barham J. P. Org. Chem. Front. 2021, 8, 1132. |
| [139] | Huang H.; Lambert T. H. Angew. Chem., Int. Ed. 2021, 60, 11163. |
| [140] | Hou Z.-W.; Yan H.; Song J.; Xu H.-C. Green Chem. 2023, 25, 7959. |
| [141] | Zhang L.; Liardet L.; Luo J.; Ren D.; Gr?tzel M.; Hu X. Nat. Catal. 2019, 2, 366. |
| [142] | Carey F. A.; Sundberg R. J. Advanced Organic Chemistry, Springer US, Boston, MA, 2007. |
/
| 〈 |
|
〉 |