

不对称催化合成手性1,2-双硼酸酯研究进展
收稿日期: 2023-12-15
修回日期: 2024-01-13
网络出版日期: 2024-01-30
基金资助
国家自然科学基金(22101177); 上海市科委(21ZR1442000); 上海市科委(23YF1426700)
Recent Advances in Catalytic Asymmetric Synthesis of Chiral 1,2-Bis(boronic) Esters
Received date: 2023-12-15
Revised date: 2024-01-13
Online published: 2024-01-30
Supported by
National Natural Science Foundation of China(22101177); Science and Technology Commission of Shanghai Municipality(21ZR1442000); Science and Technology Commission of Shanghai Municipality(23YF1426700)
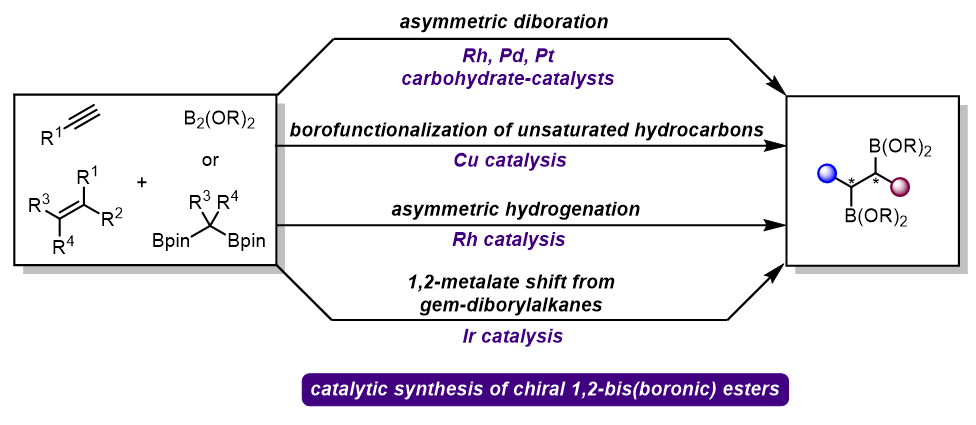
手性1,2-双硼酸酯是合成化学中重要的转化砌块, 其催化不对称合成引起了化学界的广泛关注. 近年来, 过渡金属和手性双醇催化的烯烃不对称双硼化反应已成为合成手性1,2-双硼酸酯的重要方法, 基于烯基双硼化合物的不对称氢化反应, 可以作为烯烃双硼化反应的补充来合成对应的目标产物. 同时, 烯烃或炔烃的硼化/官能团化也是构建这类化合物的另一种有效方法. 最近, 以偕二硼酸酯为起始物的催化不对称迁移增碳反应, 为手性1,2-双硼酸酯的合成提供了新的思路. 总结了手性1,2-双硼酸酯合成的最新研究进展及面临的挑战, 并对未来的研究方向进行了展望.
吉崇磊 , 高得伟 . 不对称催化合成手性1,2-双硼酸酯研究进展[J]. 有机化学, 2024 , 44(5) : 1385 -1402 . DOI: 10.6023/cjoc202312014
Chiral 1,2-bis(boronic) esters are essential building blocks in the field of synthetic chemistry, and their catalytic asymmetric synthesis has attracted significant interest of chemists. Recently, asymmetric diboration of olefins, using transition metals and chiral diols, has emerged as the straightforward and atom-economical methods for producing highly valuable chiral 1,2-bis(boronic) esters. Asymmetric hydrogenation of vinyl bis(boronic) esters can be a complementary approach to asymmetric diboration for synthesizing these products. Additionally, borofunctionalization of alkenes or alkynes represents another effective strategy for constructing these highly valuable scaffolds. A recent innovation involves catalytic asymmetric migratory coupling reactions with gem-diborylalkanes, offering new avenues for synthesizing chiral 1,2-bis(boronic) esters. The latest developements and challenges in synthesizing these moleculeshis are summarized, and the potential future research directions in this field are prospected.

| [1] | (a) Caldwell, J. J. Clin. Pharmacol. 1992, 32, 925. |
| [1] | (b) Jozwiak, K.; Lough, W. J.; Wainer, I. W. Drug Stereochemistry: Analytical Methods and Pharmacology, 3rd ed., Informa, New York, 2012. |
| [1] | (c) Rentsch, K. M. J. Biochem. Biophys. Methods 2002, 54, 1. |
| [2] | (a) Hall, D. G. Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, 2nd ed., Wiley-VCH, Weinheim, Germany, 2011. |
| [2] | (b) Trippier, P. C.; McGuigan, C. Med. Chem. Commun. 2010, 1, 183. |
| [2] | (c) Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457. |
| [2] | (d) Suzuki, A. Angew. Chem., Int. Ed. 2011, 50, 6722. |
| [2] | (e) Xu, L.; Zhang, S.; Li, P. Chem. Soc. Rev. 2015, 44, 8848. |
| [2] | (f) Brooks, W. L. A.; Sumerlin, B. S. Chem. Rev. 2016, 116, 1375. |
| [2] | (g) Diner, C.; Szabó, K. J. J. Am. Chem. Soc. 2017, 139, 2. |
| [2] | (h) Fyfe, J. W. B.; Watson, A. J. B. Chem 2017, 3, 31. |
| [2] | (i) Rygus, J. P. G.; Crudden, C. M. J. Am. Chem. Soc. 2017, 139, 18124. |
| [2] | (j) Namirembe, S.; Morken, J. P. Chem. Soc. Rev. 2019, 48, 3464. |
| [2] | (k) He, Z.; Hu, Y.; Xia, C.; Liu, C. Org. Biomol. Chem. 2019, 17, 6099. |
| [2] | (l) Kischkewitz, M.; Friese, F. W.; Studer, A. Adv. Synth. Catal. 2020, 362, 2077. |
| [2] | (m) Kalita, S. J.; Cheng, F.; Huang, Y.-Y. Adv. Synth. Catal. 2020, 362, 2778. |
| [2] | (n) Yang, K.; Song, Q. Acc. Chem. Res. 2021, 54, 2298. |
| [2] | (o) Yeung, K.; Mykura, R. C.; Aggarwal, V. K. Nat. Synth. 2022, 1, 117. |
| [2] | (p) Jiang, X.-M.; Liu, X.-R.; Chen, A.; Zou, X.-Z.; Ge, J.-F.; Gao, D.-W. Eur. J. Org. Chem. 2022, e202101463. |
| [3] | (a) Viso, A.; Fernández de la Pradilla, R.; Tortosa, M. ACS Catal. 2022, 12, 10603. |
| [3] | (b) Wang, X.; Wang, Y.; Huang, W.; Xia, C.; Wu, L. ACS Catal. 2021, 11, 1. |
| [4] | (a) Mlynarski, S. N.; Schuster, C. H.; Morken, J. P. Nature 2014, 505, 386. |
| [4] | (b) Blaisdell, T. P.; Morken, J. P. J. Am. Chem. Soc. 2015, 137, 8712. |
| [4] | (c) Crudden, C. M.; Ziebenhaus, C.; Rygus, J. P. G.; Ghozati, K.; Unsworth, P. J.; Nambo, M.; Voth, S.; Hutchinson, M.; Laberge, V. S.; Maekawa, Y.; Imao, D. Nat. Commun. 2016, 7, 11065. |
| [4] | (d) Fawcett, A.; Nitsch, D.; Ali, M.; Bateman, J. M.; Myers, E. L.; Aggarwal, V. K. Angew. Chem., Int. Ed. 2016, 55, 14663. |
| [4] | (e) Liu, X.; Sun, C.; Mlynarski, S.; Morken, J. P. Org. Lett. 2018, 20, 1898. |
| [4] | (f) Davenport, E.; Fernandez, E. Chem. Commun. 2018, 54, 10104. |
| [4] | (g) Fawcett, A.; Murtaza, A.; Gregson, C. H. U.; Aggarwal, V. K. J. Am. Chem. Soc. 2019, 141, 4573. |
| [4] | (h) Namirembe, S.; Yan, L.; Morken, J. P. Org. Lett. 2020, 22, 9174. |
| [4] | (i) Willems, S.; Toupalas, G.; Reisenbauer, J. C.; Morandi, B. A. Chem. Commun. 2021, 57, 3909. |
| [4] | (j) Mali, M.; Sharma, G. V. M.; Ghosh, S.; Roisnel, T.; Carboni, B.; Berrée, F. J. Org. Chem. 2022, 87, 7649. |
| [4] | (k) Xu, N.; Kong, Z.; Wang, J. Z.; Lovinger, G. J.; Morken, J. P. J. Am. Chem. Soc. 2022, 144, 17815. |
| [4] | (l) Zhang, M.; Lee, P. S.; Allais, C.; Singer, R. A.; Morken, J. P. J. Am. Chem. Soc. 2023, 145, 8308. |
| [5] | Ma, X.; Murray, B.; Biscoe, M. R. Nat. Rev. Chem. 2020, 4, 584. |
| [6] | Blair, D. J.; Tanini, D.; Bateman, J. M.; Scott, H. K.; Myers, E. L.; Aggarwal, V. K. Chem. Sci. 2017, 8, 2898. |
| [7] | (a) Coombs. J. R.; Morken. J. P. Angew. Chem., Int. Ed. 2016, 55, 2636. |
| [7] | (b) Obligacion, J. V.; Chirik, P. J. Nat. Rev. Chem. 2018, 2, 15. |
| [7] | (c) Wang, F.; Chen, P.; Liu, G. Acc. Chem. Res. 2018, 51, 2036. |
| [7] | (d) Chen, J.-H.; Guo. J.; Lu, Z. Chin. J. Chem. 2018, 36, 1075. |
| [7] | (e) Li, Z.-L.; Fang, G.-C.; Gu, Q.-S.; Liu, X.-Y. Chem. Soc. Rev. 2020, 49, 32. |
| [8] | Morgan, J. B.; Miller, S. P.; Morken, J. P. J. Am. Chem. Soc. 2003, 125, 8702. |
| [9] | Miller, S. P.; Morgan, J. B.; Nepveux, V. F. J.; Morken, J. P. Org. Lett. 2004, 6, 131. |
| [10] | Trudeau, S.; Morgan, J. B.; Shrestha, M.; Morken, J. P. J. Org. Chem. 2005, 70, 9538. |
| [11] | Toribatake, K.; Nishiyama, H. Angew. Chem., Int. Ed. 2013, 52, 11011. |
| [12] | Pelz, N. F.; Woodward, A. R.; Burks, H. E.; Sieber, J. D.; Morken, J. P. J. Am. Chem. Soc. 2004, 126, 16328. |
| [13] | Sieber, J. D.; Morken, J. P. J. Am. Chem. Soc. 2006, 128, 74. |
| [14] | Woodward, A. R.; Burks, H. E.; Chan, L. M.; Morken, J. P. Org. Lett. 2005, 7, 5505. |
| [15] | Pelz, N. F.; Morken, J. P. Org. Lett. 2006, 8, 4557. |
| [16] | Kliman, L. T.; Mlynarski, S. N.; Ferris, G. E.; Morken, J. P. J. Am. Chem. Soc. 2009, 131, 13210. |
| [17] | (a) Kliman, L. T.; Mlynarski, S. N.; Morken, J. P. Angew. Chem., Int. Ed. 2012, 51, 512. |
| [17] | (b) Coombs, J. R.; Haeffner, F.; Kliman, L. T.; Morken, J. P. J. Am. Chem. Soc. 2013, 135, 11222. |
| [18] | Ferris, G. E.; Hong, K.; Roundtree, I. A.; Morken, J. P. J. Am. Chem. Soc. 2013, 135, 2501. |
| [19] | Coombs, J. R.; Zhang, L.; Morken, J. P. J. Am. Chem. Soc. 2014, 136, 16140. |
| [20] | (a) Byrom, N. T.; Grigg, R.; Kongkathip, B.; Reimer, G.; Wade, A. R. J. Chem. Soc., Perkin Trans. 1 1984, 1643. |
| [20] | (b) Page, P. C. B.; Rayner, C. M.; Sutherland, I. O. Tetrahedron Lett. 1986, 27, 3535. |
| [20] | (c) Page, P. C. B.; Rayner, C. M.; Sutherland, I. O. J. Chem. Soc., Chem. Commun. 1988, 356. |
| [20] | (d) Page, P. C. B.; Rayner, C. M.; Sutherland, I. O. J. Chem. Soc., Perkin Trans. 1 1990, 1375. |
| [20] | (e) Mayer, S. F.; Mang, H.; Steinreiber, A.; Saf, R.; Faber, K. Can. J. Chem. 2002, 80, 362. |
| [21] | Nóvoa, L.; Trulli, L.; Parra, A.; Tortosa, M. Angew. Chem., Int. Ed. 2021, 60, 11763. |
| [22] | (a) Bonet, A.; Sole, C.; Gulyás, H.; Fernández, E. Org. Biomol. Chem. 2012, 10, 6621. |
| [22] | (b) Bonet, A.; Pubill-Ulldemolins, C.; Bo, C.; Gulyás, H.; Fernández, E. Angew. Chem., Int. Ed. 2011, 50, 7158. |
| [23] | Fang, L.; Yan, L.; Haeffner, F.; Morken, J. P. J. Am. Chem. Soc. 2016, 138, 2508. |
| [24] | Yan, L.; Meng, Y.; Haeffner, F.; Leon, R. M.; Crockett, M. P.; Morken, J. P. J. Am. Chem. Soc. 2018, 140, 3663. |
| [25] | Yan, L.; Morken, J. P. Org. Lett. 2019, 21, 3760. |
| [26] | Lee, Y.; Jang, H.; Hoveyda, A. H. J. Am. Chem. Soc. 2009, 131, 18234. |
| [27] | Lee, Y.; Hoveyda, A. H. J. Am. Chem. Soc. 2009, 131, 3160. |
| [28] | Jung, H.-Y.; Yun, J. Org. Lett. 2012, 14, 2606. |
| [29] | Zanghi, J. M.; Liu, S.; Meek, S. J. Org. Lett. 2019, 21, 5172. |
| [30] | Radomkit, S.; Liu, Z.; Closs, A.; Mikus, M. S.; Hoveyda, A. H. Tetrahedron 2017, 73, 5011. |
| [31] | Lee, H.; Lee, S.; Yun, J. ACS Catal. 2020, 10, 2069. |
| [32] | Green, J. C.; Joannou, M. V.; Murray, S. A.; Zanghi, J. M.; Meek, S. J. ACS Catal. 2017, 7, 4441. |
| [33] | Fan, Z.; Ye, M.; Wang, Y.; Qiu, J.; Li, W.; Ma, X.; Yang, K.; Song, Q. ACS Cent. Sci. 2022, 8, 1134. |
| [34] | Morgan, J. B.; Morken, J. P. J. Am. Chem. Soc. 2004, 126, 15338. |
| [35] | Paptchikhine, A.; Cheruku, P.; Engman, M.; Andersson, P. G. Chem. Commun. 2009, 5996. |
| [36] | (a) Allen, A. E.; MacMillan, D. W. C. Chem. Sci. 2012, 3, 633. |
| [36] | (b) Pye, D. R.; Mankad, N. P. Chem. Sci. 2017, 8, 1705. |
| [36] | (c) Fu, J.; Huo, X.; Li, B.; Zhang, W. Org. Biomol. Chem. 2017, 15, 9747. |
| [36] | (d) Kim, U. B.; Jung, D. J.; Jeon, H. J.; Rathwell, K.; Lee, S.-g. Chem. Rev. 2020, 120, 13382. |
| [36] | (e) Tian, F.; Zhang, J.; Yang, W.-L.; Deng, W.-P. Chin. J. Org. Chem. 2020, 40, 3262. |
| [36] | (f) Huo, X.; Li, G.; Wang, X.; Zhang, W. Angew. Chem., Int. Ed. 2022, 61, e202210086. |
| [36] | (g) Wei, L.; Wang, C.-J. Chin. J. Chem. 2021, 39, 15. |
| [36] | (h) Martínez, S.; Veth, L.; Lainer, B.; Dydio, P. ACS Catal. 2021, 11, 3891. |
| [36] | (i) Wei, L.; Wang, C.-J. Chem. Catal. 2023, 3, 100455. |
| [37] | (a) Wang, Y.; Liu, X.; Deng, L. J. Am. Chem. Soc. 2006, 128, 3928. |
| [37] | (b) Wang, B.; Wu, F.; Wang, Y.; Liu, X.; Deng, L. J. Am. Chem. Soc. 2007, 129, 768. |
| [37] | (c) Zhu, B.; Lee, R.; Li, J.; Ye, X.; Hong, S.-N.; Qiu, S.; Coote, M. L.; Jiang, Z. Angew. Chem., Int. Ed. 2016, 55, 1299. |
| [37] | (d) Li, Z.; Hu, B.; Wu, Y.; Fei, C.; Deng, L. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 1730. |
| [37] | (e) Trost, B. M.; Zell, D.; Hohn, C.; Mata, G.; Maruniak, A. Angew. Chem., Int. Ed. 2018, 57, 12916. |
| [37] | (f) Trost, B. M.; Schultz, J. E.; Chang, T.; Maduabum, M. R. J. Am. Chem. Soc. 2019, 141, 9521. |
| [37] | (g) Yang, S.-Q.; Wang, Y.-F.; Zhao, W.-C.; Lin, G.-Q.; He, Z.-T. J. Am. Chem. Soc. 2021, 143, 7285. |
| [37] | (h) Zhang, J.; Huo, X.; Xiao, J.; Zhao, L.; Ma, S.; Zhang, W. J. Am. Chem. Soc. 2021, 143, 12622. |
| [37] | (i) Dai, J.; Li, L.; Ye, R.; Wang, S.; Wang, Y.; Peng, F.; Shao, Z. Angew. Chem., Int. Ed. 2023, 62, e202300756. |
| [38] | (a) Miralles, N.; Maza, R. J.; Fernández, E. Adv. Synth. Catal. 2018, 360, 1306. |
| [38] | (b) Nallagonda, R.; Padala, K.; Masarwa, A. Org. Biomol. Chem. 2018, 16, 1050. |
| [38] | (c) Wu, C.; Wang, J. Tetrahedron Lett. 2018, 59, 2128. |
| [38] | (d) Cuenca, A. B.; Fernández, E. Chem. Soc. Rev. 2021, 50, 72. |
| [38] | (e) Corro, M.; Salvado, O.; González, S.; Dominguez-Molano, P.; Fernández, E. Eur. J. Inorg. Chem. 2021, 2802. |
| [38] | (f) Jo, W.; Lee, J. H.; Cho, S. H. Chem. Commun. 2021, 57, 4346. |
| [38] | (g) Lee, Y.; Han, S.; Cho, S. H. Acc. Chem. Res. 2021, 54, 3917. |
| [38] | (h) Zhang, C.; Hu, W.; Morken, J. P. ACS Catal. 2021, 11, 10660. |
| [38] | (i) Paul, S.; Das, K. K.; Aich, D.; Manna, S.; Panda, S. Org. Chem. Front. 2022, 9, 838. |
| [39] | (a) Zhang, L.; Lovin?er, G. J.; Edelstein, E. K.; Szymaniak, A. A.; Chierchia, M. P.; Morken, J. P. Science 2016, 351, 70. |
| [39] | (b) Lovinger, G. J.; Aparece, M. D.; Morken, J. P. J. Am. Chem. Soc. 2017, 139, 3153. |
| [39] | (c) Chierchia, M.; Law, C.; Morken, J. P. Angew. Chem., Int. Ed. 2017, 56, 11870. |
| [39] | (d) Zhang, X.; Gao, C.; Morken, J. P. J. Am. Chem. Soc. 2023, 145, 16344. |
| [39] | (e) For a review, see: Namirembe, S.; Morken, J. P. Chem. Soc. Rev. 2019, 48, 3464. |
| [40] | (a) Davis, C. R.; Luvaga, I. K.; Ready, J. M. J. Am. Chem. Soc. 2021, 143, 4921. |
| [40] | (b) Davis, C. R.; Fu, Y.; Liu, P.; Ready, J. M. J. Am. Chem. Soc. 2022, 144, 16118. |
| [41] | (a) Ge, J.-F.; Zou, X.-Z.; Liu, X.-R.; Ji, C.-L.; Zhu, X.-Y.; Gao, D.-W. Angew. Chem., Int. Ed. 2023, 62, e202307447. |
| [41] | (b) Chen, A.; Qiao, Y.; Gao, D.-W. Angew. Chem., Int. Ed. 2023, 62, e202312605. |
| [42] | Jiang, X.-M.; Ji, C.-L.; Ge, J.-F.; Zhao, J.-H.; Zhu, X.-Y.; Gao, D.-W. Angew. Chem., Int. Ed. 2024, 63, e202318441. |
/
| 〈 |
|
〉 |