

邻二碘芳烃和氢化钠产生芳炔用于硫醇的邻碘芳基化反应
收稿日期: 2023-12-23
修回日期: 2024-03-08
网络出版日期: 2024-03-20
基金资助
国家自然科学基金(22271206); 国家自然科学基金(22071053); 江苏省高校重点学科建设(PAPD); 苏州市脑病诊疗国际联合实验室资助项目
o-Iodoarylation of Thiols Enabled by Aryne Generated from o-Diiodoarene and Sodium Hydride
Received date: 2023-12-23
Revised date: 2024-03-08
Online published: 2024-03-20
Supported by
National Natural Science Foundation of China(22271206); National Natural Science Foundation of China(22071053); Priority Academic Program Development of Jiangsu Higher Education Institutions(PAPD); Suzhou International Joint Laboratory for Diagnosis and Treatment of Brain Diseases.
刘典范 , 王健智 , 王梦晴 , 陈晓蓓 , 胡延维 , 张士磊 . 邻二碘芳烃和氢化钠产生芳炔用于硫醇的邻碘芳基化反应[J]. 有机化学, 2024 , 44(7) : 2363 -2370 . DOI: 10.6023/cjoc202312022
A simple and efficient method is described for the synthesis of o-iodoaryl thioethers by the reaction of thiols and aryne. The aryne is generated from inexpensive and readily available reagents o-diiodoarene and sodium hydride. Remarkably, no disulfide substituted byproduct was observed in the reactions which is different from transition metal-catalyzed process. This method features simple operation, mild reaction conditions, broad substrate scope, and high reaction yields for many cases.
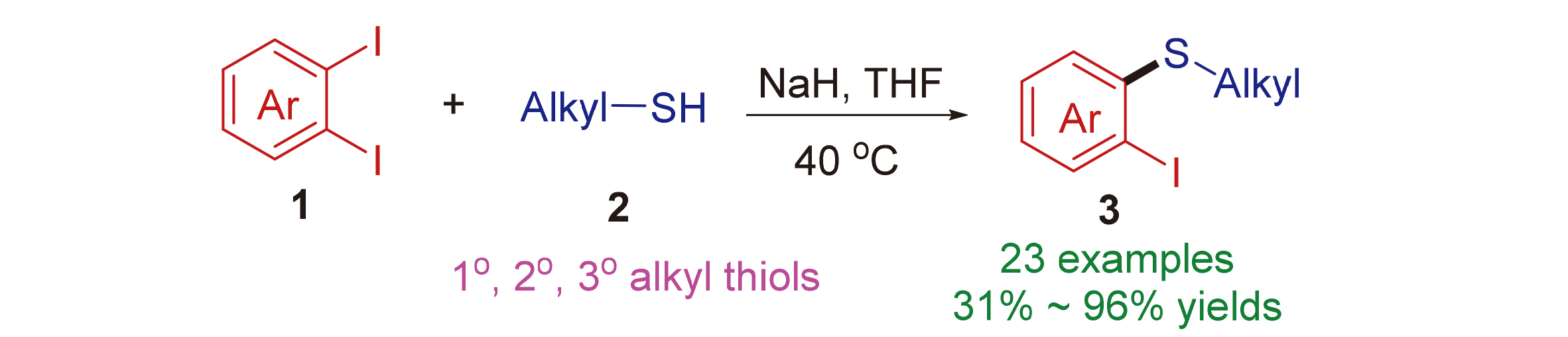
Key words: aryne; thiol; o-diiodoarene; sodium hydride; o-iodoaryl thioether
| [1] | Wang, N.; Saidhareddya, P.; Jiang, X. Nat. Prod. Rep. 2020, 37, 246. |
| [2] | Liu, H.; Jiang, X. Chem. Asian J. 2013, 8, 2546. |
| [3] | Dunbar, K. L.; Scharf, D. H.; Litomska, A.; Hertweck, C. Chem. Rev. 2017, 117, 5521. |
| [4] | Criscieli, B.; Beatriz, S.; Caren, S.; George, B.; Beatriz, S.; Nelson, C. Curr. Org. Synth. 2020, 17, 192. |
| [5] | Wu, Q.; Bell, B. A.; Yan, J. X.; Chevrette, M. G.; Brittin, N. J.; Zhu, Y.; Chanana, S.; Maity, M.; Braun, D. R.; Wheaton, A. M.; Guzei, I. A.; Ge, Y.; Rajski, S. R.; Thomas, M. G.; Bugni, T. S. J. Am. Chem. Soc. 2023, 145, 58. |
| [6] | Cinar, M. E.; Ozturk, T. Chem. Rev. 2015, 115, 3036. |
| [7] | Zhang, M.; Zhang, B. B.; Lin, Q.; Jiang, Z.; Zhang, J.; Li, Y.; Pei, S.; Han, X.; Xiong, H.; Liang, X.; Lin, Y.; Wei, Z.; Zhang, F.; Zhang, X.; Wang, Z.-X.; Shi, Q.; Huang, H. Angew. Chem., Int. Ed. 2023, 62, e202306307. |
| [8] | Lou, J.; Wang, Q.; Wu, P.; Wang, H.; Zhou, Y.-G.; Yu, Z. Chem. Soc. Rev. 2020, 49, 4307. |
| [9] | Lin, Y.; Li, Z.; Ma, H.; Wang, Y.; Wang, X.; Song, S.; Zhao, L.; Wu, S.; Tian, S.; Fu, C.; Luo, L.; Zhu, F.; He, S.; Zheng, J.; Zhang, X. Chem. Med. Chem. 2020, 15, 1150. |
| [10] | Ji, X.; Li, Z. Med. Res. Rev. 2020, 40, 1519. |
| [11] | Huang, X.-L.; Chen, J.-L.; Li, X.-L.; Zhao, L.; Cui, Y.-D.; Liu, J.-Y.; Morris-Natschke, S. L.; Masuo, G.; Cheng, Y.-Y.; Lee, K.-H.; Chen, D.-F.; Zhang, J. J. Asian Nat. Prod. Res. 2021, 23, 703. |
| [12] | Beletskaya, I. P.; Ananikov, V. P. Chem. Rev. 2022, 122, 16110. |
| [13] | Beletskaya, I. P.; Ananikov, V. P. Chem. Rev. 2011, 111, 1596. |
| [14] | Ghaderi, A. Tetrahedron 2016, 72, 4758. |
| [15] | Sundaravelu, N.; Sangeetha, S.; Sekar, G. Org. Biomol. Chem. 2021, 19, 1459. |
| [16] | Kanchana, U. S.; Diana, E. J.; Mathew, T. V. Asian J. Org. Chem. 2022, 11, e202200038. |
| [17] | Lee, C. F.; Liu, Y. C.; Badsara, S. S. Chem. Asian J. 2014, 9, 706. |
| [18] | Zhao, S.; Chen, K.; Zhang, L.; Yang, W.; Huang, D. Adv. Synth. Catal. 2020, 362, 3516. |
| [19] | Bag, R.; Sharma, N. K. Org. Chem. Front. 2023, 10, 1252. |
| [20] | Chaitanya, M.; Anbarasan, P. Org. Lett. 2018, 20, 3362. |
| [21] | Zhang, G.; Liu, C.; Yi, H.; Meng, Q.; Bian, C.; Chen, H.; Jian, J.-X.; Wu, L.-Z.; Lei, A. J. Am. Chem. Soc. 2015, 137, 9273. |
| [22] | Li, M.; Wang, J. J. Org. Lett. 2018, 20, 6490. |
| [23] | Kang, Y.-S.; Zhang, P.; Li, M.-Y.; Chen, Y.-K.; Xu, H.-J.; Zhao, J.; Sun, W.-Y.; Yu, J.-Q.; Lu, Y. Angew. Chem., Int. Ed. 2019, 58, 9099. |
| [24] | Fernandes, R. A.; Bhowmik, A.; Yadav, S. S. Org. Biomol. Chem. 2020, 18, 9583. |
| [25] | Sun, N.; Zheng, K.; Zhang, M.; Zheng, G.; Jin, L.; Hu, B.; Shen, Z.; Hu, X. Green Chem. 2023, 25, 2782. |
| [26] | Song, C.; Liu, K.; Dong, X.; Chiang, C.-W.; Lei, A. Synlett 2019, 30,1149. |
| [27] | Yang, D.; Yan, Q.; Zhu, E.; Lv, J.; He, W. M. Chin. Chem. Lett. 2022, 33, 1798. |
| [28] | Annamalai, P.; Liu, K.-C.; Badsara, S. S.; Lee, C.-F. Chem. Rec. 2021, 21, 3674. |
| [29] | Cabrera‐Afonso, M. J.; Granados, A.; Molander, G. A. Angew. Chem., Int. Ed. 2022, 61, e202202706. |
| [30] | Zhang, M.; Wang, B.; Cao, Y.; Liu, Y.; Wang, Z.; Wang, Q. Org. Lett. 2022, 24, 8895. |
| [31] | Pramanik, M.; Choudhuri, K.; Mal, P. Org. Biomol. Chem. 2020, 18, 8771. |
| [32] | Saroha, M.; Sindhu, J.; Kumar, S.; Bhasin, K. K.; Khurana, J. M.; Varma, R. S.; Tomar, D. ChemistrySelect 2021, 6, 13077. |
| [33] | Chen, S.; Wen, Q.; Zhu, Y.; Ji, Y.; Pu, Y.; Liu, Z.; He, Y.; Feng, Z. Chin. Chem. Lett. 2022, 33, 5101. |
| [34] | Yang, P.; Zheng, C.; Nie, Y. H.; You, S. L. Chem. Sci. 2020, 11, 6830. |
| [35] | Zhu, X.; Li, W.; Luo, X.; Deng, G.; Liang, Y.; Liu, J. Green Chem. 2018, 20, 1970. |
| [36] | Li, X.; Zhou, B.; Yang, R.-Z.; Yang, F.-M.; Liang, R.-X.; Liu, R.-R.; Jia, Y.-X. J. Am. Chem. Soc. 2018, 140, 13945. |
| [37] | Flynn, A. R.; McDaniel, K.; Hughes, M.; Vogt, D.; Jui, N. T. J. Am. Chem. Soc. 2020, 142, 9163. |
| [38] | Zhou, B.; Wang, H.; Cao, Z.-Y.; Zhu, J.-W.; Liang, R.-X.; Hong, X.; Jia, Y.-X. Nat. Commun. 2020, 11, 4380. |
| [39] | Liu, W.; Min, H.; Zhu, X.; Deng, G.; Liang, Y. Org. Biomol. Chem. 2017, 15, 9804. |
| [40] | Li, L.; Liu, X.-L.; Qi, Z.; Yang, A.-H.; Ma, A.-J.; Peng, J.-B. Org. Lett. 2022, 24, 1201. |
| [41] | Ca, N. D.; Fontana, M.; Motti, E.; Catellani, M. Chem. Res. 2016, 49, 1389. |
| [42] | Wang, J.; Dong, G. Chem. Rev. 2019, 119, 7478. |
| [43] | Liu, J.; Lin, H.; Jiang, H.; Huang, L. Org. Lett. 2022, 24, 484. |
| [44] | Zeng, Y.; Li, G.; Hu, J. Angew. Chem., Int. Ed. 2015, 54, 10773. |
| [45] | Zeng, Y.; Zhang, L.; Zhao, Y.; Ni, C.; Zhao, J.; Hu, J. J. Am. Chem. Soc. 2013, 135, 2955. |
| [46] | Cao, W.; Niu, S. L.; Shuai, L.; Xiao, Q. Chem. Commun. 2020, 56, 972. |
| [47] | Wang, W.; Ding, M.; Zhao, C. G.; Chen, S.; Zhu, C.; Han, J.; Li, W.; Xie, J. Angew. Chem., Int. Ed. 2023, 62, e202304019. |
| [48] | Cao, W. X.; Zhu, L.; He, Y.; Wang, R.; Liu, M.; Ouyang, Q.; Xiao, Q. Angew. Chem., Int. Ed. 2023, e202305146. |
| [49] | Chen, D.; Yang, C.; Li, M.; Zhao, G.; Wang, W.; Wang, X.; Quan, Z. Chin. J. Org. Chem. 2023, 43, 503. (in Chinese) |
| [49] | (陈东平, 杨春红, 李明, 赵国孝, 王文鹏, 王喜存, 权正军, 有机化学, 2023, 43, 503.) |
| [50] | Yan, Q.; Fan, R.; Liu, B.; Su, S.; Wang, B.; Yao, T.; Tan, J. Chin. J. Org. Chem. 2021, 41, 455. (in Chinese) |
| [50] | (闫强, 范荣, 刘斌斌, 苏帅松, 王勃, 姚团利, 谭嘉靖, 有机化学, 2021, 41, 455.) |
| [51] | Liu, Y.; Wang, H.; Cao, X.; Fang, Z.; Wan, J. P. Synthesis 2013, 45, 2977. |
| [52] | Carril, M.; SanMartin, R.; Domínguez, E.; Tellitu, I. Chem.-Eur. J. 2007, 13, 5100. |
| [53] | Reinhard, D. L.; Heinen, F.; Stoesser, J.; Engelage, E.; Huber, S. M. Helv. Chim. Acta 2021, 104, e2000221. |
| [54] | Luo, F.; Li, C.; Ji, P.; Zhou, Y.; Gui, J.; Chen, L.; Yin, Y.; Zhang, X; Hu, Y.; Chen, X.; Liu, X.; Chen, X.; Yu, Z.; Wang, W.; Zhang, S. Chem 2023, 9, 2620. |
| [55] | Jiang, Y.; Zhu, W.; Huang, J.; Luo, F.; Chen, X.; Fang, C.; Chen, X.; Liu, S.; Hu, Y.; Zhang, S. Org. Chem. Front. 2024, 11, 12. |
| [56] | Hu, M.; Liu, D.; Liu, Y.; Luo, F.; Chen, X.; Yin, Y.; Zhang, S.; Hu, Y. Adv. Synth. Catal. 2024, 366, 1538. |
| [57] | Liu, Y.; Mao, Y.; Hu, Y.; Gui, J.; Wang, L.; Wang, W.; Zhang, S. Adv. Synth. Catal. 2019, 361, 1554. |
| [58] | Sun, W.; Chen, X.; Hu, Y.; Geng, H.; Jiang, Y.; Zhou, Y.; Zhu, W.; Hu, M.; Hu, H.; Wang, X.; Wang, X.; Zhang, S.; Hu, Y. Tetrahedron Lett. 2020, 61, 152442. |
| [59] | Gui, J.; Cai, X.; Chen, L.; Zhou, Y.; Zhu, W.; Jiang, Y.; Hu, M.; Chen, X.; Hu, Y.; Zhang, S. Org. Chem. Front. 2021, 8, 4685. |
| [60] | Luo, F.; Chen, X.; Yu, J.; Yin, Y.; Hu, X.; Hu, Y.; Liu, X.; Chen, X.; Zhang, S.; Hu, Y. Synthesis 2023, 55, 1451. |
| [61] | Mao, Y.; Liu, Y.; Hu, Y.; Wang, L.; Zhang, S.; Wang, W. ACS Catal. 2018, 8, 3016. |
| [62] | Leroux, F.; Schlosser, M. Angew. Chem., Int. Ed. 2002, 41, 4272. |
| [63] | Leroux, F. R.; Bonnafoux, L.; Heiss, C.; Colobert, F.; Lanfranchi, D. A. Adv. Synth. Catal. 2007, 349, 2705. |
| [64] | Bonnafoux, L.; Colobert, F.; Leroux, F. R. Synlett 2010, 2953. |
| [65] | Diemer, V.; Begaud, M.; Leroux, F. R.; Colobert, F. Eur. J. Org. Chem. 2011, 341. |
| [66] | Berthelot-Brehier, A.; Panossian, A.; Colobert, F.; Leroux, F. R. Org. Chem. Front. 2015, 2, 634. |
/
| 〈 |
|
〉 |