

铜基固体废弃物促进的末端炔烃交叉偶联反应
收稿日期: 2024-01-12
修回日期: 2024-03-07
网络出版日期: 2024-03-28
基金资助
国家自然科学基金(21961037); 国家自然科学基金(22161044); 国家自然科学基金(22201241); 国家自然科学基金(22361044); 天山科技创新领军人才计划(2022TSYCLJ0016); 新疆维吾尔自治区自然科学基金重点(2022D01D06)
Copper-Based Solid Wastes Promoted Cross-Coupling Reactions of Terminal Alkynes
Received date: 2024-01-12
Revised date: 2024-03-07
Online published: 2024-03-28
Supported by
National Natural Science Foundation of China(21961037); National Natural Science Foundation of China(22161044); National Natural Science Foundation of China(22201241); National Natural Science Foundation of China(22361044); Tianshan Talents Program for Leading Talents in Science and Technology Innovation(2022TSYCLJ0016); Key Program of Natural Science Foundation of Xinjiang Uygur Autonomous Region(2022D01D06)
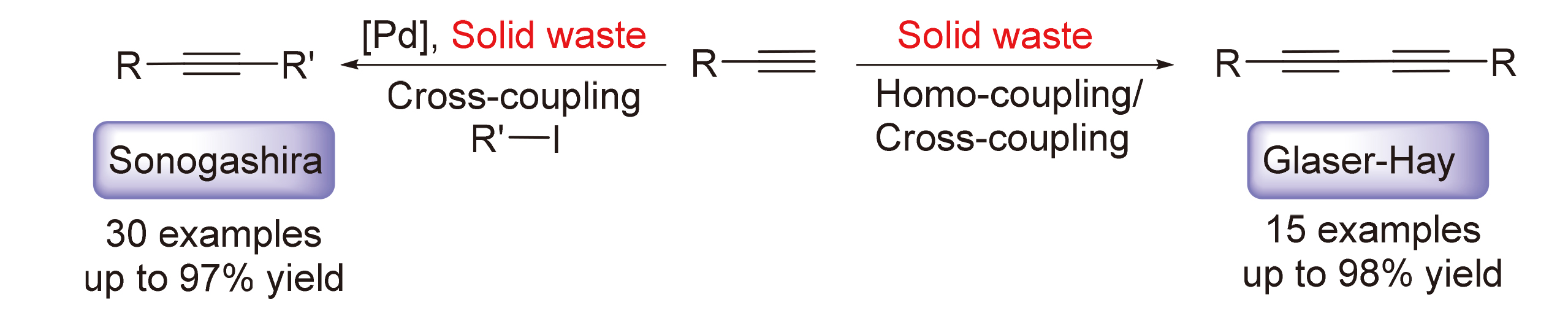
开发了一种使用铜基固体废弃物代替传统铜盐参与Sonogashira和Glaser-Hay偶联反应的方法, 在较为温和的条件下, 高效地合成了多种二取代炔烃. 对于Sonogashira偶联反应, 各种取代的苯乙炔和碘代芳烃反应效果良好, 以57%~97%的产率生成二芳基乙炔类化合物. 对于Glaser-Hay偶联反应, 多种末端炔烃既能以优秀的产率生成对称二取代1,3-丁二炔衍生物, 也能以中等的产率合成交叉偶联产物. 克级规模实验和循环实验表明该固体废弃物在有机合成中有潜在的应用前景.
关键词: 铜基固体废弃物; 末端炔烃; Sonogashira偶联反应; Glaser-Hay偶联反应
徐童 , 张宁 , 张永红 , 王斌 , 夏昱 , 金伟伟 , 金聘入 , 刘晨江 . 铜基固体废弃物促进的末端炔烃交叉偶联反应[J]. 有机化学, 2024 , 44(7) : 2341 -2349 . DOI: 10.6023/cjoc202401012
An approach to the Sonogashira and Glaser-Hay coupling reactions utilizing copper-based solid wastes instead of traditional copper salts has been developed. This method enables the efficient synthesis of various disubstituted alkynes under relatively mild conditions. For the Sonogashira coupling reaction, diverse substituted phenylacetylenes and aryl iodides exhibit excellent reactivity, resulting in diaryl acetylene compounds with yields ranging from 57% to 97%. The Glaser-Hay coupling reaction allows for the high-yield production of symmetrically disubstituted 1,3-butadiyne derivatives using a variety of terminal alkynes. Additionally, moderate yields can be achieved for cross-coupling products. Gram-scale and cycling experiments demonstrate promising prospects for the application of solid waste in organic synthesis.

| [1] | (a) Chinchilla, R.; Carmen Najera, C. Chem. Rev. 2014, 114, 1783. |
| [1] | (b) Stang, P. J.; Diederich, F. Modern Acetylene Chemistry, VCH, Weinheim, 1995. |
| [1] | (c) Diederich, F.; Stang, P. J. Acetylene Chemistry, Ed.: Tykwinski, R. R., Wiley-VCH, Weinheim, 2005. |
| [1] | (d) Feng, J.; Zhang, F; Shu, C.-Y.; Zhu, G.-G. Chin. J. Chem. 2022, 40, 1667. |
| [1] | (e) Hao, T.-G.; Shi, M.; Wei, Y. Chin. J. Chem. 2023, 41, 301. |
| [1] | (f) Batchu, V. R.; Subramanian, V.; Parasuraman, K.; Swamy, N. K.; Kumar, S.; Pal, M. Tetrahedron 2005, 61, 9869. |
| [2] | (a) Armstrong, K. M.; Lalic, G. J. Am. Chem. Soc. 2019, 141, 6173. |
| [2] | (b) Hamasaka, G.; Roy, D.; Tazawa, A.; Uozumi, Y. ACS Catal. 2019, 9, 11640. |
| [2] | (c) Liu, L.; Dan Zhou, D.; Liu, M.; Zhou, Y.-B.; Chen, T.-Q. Org. Lett. 2018, 20, 2741. |
| [2] | (d) Tian, Z.-Y.; Wang, S.-M.; Jia, S.-J.; Song, H.-X.; Zhang, C.-P. Org. Lett. 2017, 19, 5454. |
| [2] | (e) Liu, B.-Q.; Yan, Z.-F.; Quan, Z.-J. Chin. J. Org. Chem. 2018, 38, 3032. (in Chinese) |
| [2] | (刘伯渠, 燕中飞, 权正军, 有机化学, 2018, 38, 3032.) |
| [3] | (a) Ma, N.; Zeng, X.-H. Chin. J. Org. Chem. 2018, 38, 1556. (in Chinese) |
| [3] | (马楠, 曾祥华, 有机化学, 2018, 38, 1556.) |
| [3] | (b) Li, X.-W.; Liu, X.-H.; Chen, H.-J.; Wu, W.-Q.; Qi, C.-F.; Jiang, H.-F. Angew. Chem., Int. Ed. 2014, 53, 14485. |
| [3] | (c) Sakamoto, R.; Kato, T.; Sakurai, S.; Maruoka, K. Org. Lett. 2018, 20, 1400. |
| [3] | (d) Feng, L.-L.; Hu, T.-J.; Zhang, S.-S.; Xiong, H.-Y.; Zhang, G.-W. Org. Lett. 2019, 21, 9487. |
| [3] | (e) Lv, Y.-H.; Pu, W.-Y.; Shi, L.-H. Org. Lett. 2019, 21, 6034. |
| [3] | (f) Biswas, S.; Mullick, K.; Chen, S.-Y.; Kriz, D. A.; Shakil, M.; Kuo, C.-H.; Angeles-Boza, A. M.; Rossi, A. R.; Suib, S. L. ACS Catal. 2016, 6, 5069. |
| [4] | (a) Zhang, G.; Shao, X.-B.; Li, Q.-H.; Yang, X.-J. Chin. J. Org. Chem. 2018, 38, 1538. (in Chinese) |
| [4] | (张刚, 杓学蓓, 李清寒, 杨学军, 有机化学, 2018, 38, 1538.) |
| [4] | (b) Chen, H.; Yao, L.-C.; Guo, L.; Liu, Y. A.; Tian, B.-X.; Liao, X.-B. Cell Rep. Phys. Sci. 2023, 4, 101573. |
| [4] | (c) Li, Y.-Q.; Li, F.; Shi, S.-L. Chin. J. Chem. 2020, 38, 1035. |
| [5] | Islam, K. M. N. Renewable Sustainable Energy Rev. 2018, 81, 2472. |
| [6] | Martins, M. A. D. B.; Crispim, A.; Ferreira, M. L.; Dos Santos, I. F.; Melo, M. D. L. N.; Barros, R. M.; Filho, G. L. T. Cleaner Waste Systems 2023, 4, 100070 |
| [7] | Ding, Y.; Zhao, J.; Liu, J.; Zhou, J.; Cheng, L.; Zhao, J.; Shao, Z.; Iris, ?.; Pan, B.; Li, X.; Hu, Z. J. Clean. Prod. 2021, 293, 126144. |
| [8] | (a) Yu, H.-X.; Zahidi, I.; Liang, D.-F. J. Mater. Res. Technol. 2023, 23, 5733. |
| [8] | (b) Arenas, C.; Ríos, D. J.; Cifuentes, H.; Vilches, F. L.; Leiva, C. Eur. J. Environ. Civ. Eng. 2022, 9, 3805. |
| [8] | (c) Zhang, F.; Yu, W.; Liu, W.-Y.; Xu, Z.-Y. Front. Energy Res. 2020, 8, 50. |
| [8] | (d) Liu, B.-C.; Han, B.-R.; Liang, X.-Q.; Liu, Y.-F. Int. J. Hydrogen Energy 2024, 52, 1445. |
| [9] | (a) Dewan, A.; Sarmah, M.; Bora, U.; Thakur, A. J. Tetrahedron Lett. 2016, 57, 3760. |
| [9] | (b) Dewan, A.; Sarmah, M.; Bora, U.; Thakur, A. J. Appl. Organomet. Chem. 2017, 31, e3646. |
| [9] | (c) Dewan, A.; armah, M.; Thakur, A. J.; Bharali, P.; Bora, U. ACS Omega 2018, 3, 5327. |
| [9] | (d) Boruah, P.-R.; Ali, A.-A.; Saikia, B.; Sarma, D. Green Chem. 2015, 17, 1442. |
| [10] | (a) Isfahani, A. L.; Mohammadpoor-Baltork, I.; Mirkhani, V.; hosropour, A. R.; Moghadam, M.; Tangestaninejad, S. Eur. J. Org. Chem. 2014, 5603. |
| [10] | (b) Thathagar, M. B.; Beckers, J.; Rothenberg, G. Green Chem. 2004, 6, 215. |
| [10] | (c) Zhang, C.-T.; Peng, L.-J.; Song, B.-H.; Li, Z.-W.; Cao, X.-Q. Inorg. Chem. Commun. 2023, 158, 111471. |
| [10] | (d) Moghaddam, F. M.; Tavakoli, G.; Rezvani, H. R. Catal. Commun. 2015, 60, 82. |
| [10] | (e) Mohammadi, P.; Heravil, M. M.; Mohammadi, L.; Saljooqi, A. Sci. Rep. 2023, 13, 17375. |
| [11] | (a) Tang, S.-Y.; Li, L.-J.; Ren, X.-H.; Li, J.; Yang, G.-Y.; Li, H.; Yuan, B.-X. Green Chem. 2019, 21, 2899. |
| [11] | (b) Su, L.-B.; Dong, J.-Y.; Liu, L.; Sun, M.-L.; Qiu, R.-H.; Zhou, Y.-B.; Yin, S.-F. J. Am. Chem. Soc. 2016, 138, 12348. |
| [11] | (c) Kusuda, A.; Xu, X.-X.; Wang, X.; Tokunaga, E.; Shibata, N. Green Chem. 2011, 13, 843. |
| [11] | (d) Zhang, L.-Z.; Wei, C.-B.; Wu, J.-W.; Liu, D.; Yao, Y.-C.; Chen, Z.; Liu, J.-X.; Yao, C.-J.; Li, D.-H.; Yang, R.-J.; Xia, Z.-H. Chem. Sci. 2022, 13, 7475. |
| [12] | (a) Sun, Y.-J.; Wang, R.; Liu, T.-X.; Jin, W.-W.; Wang, B.; Zhang, Y.-H.; Xia, Y.; Liu, C.-J. Eur. J. Org. Chem. 2021, 2470. |
| [12] | (b) Sun, Y.-J.; Jin, W.-W.; Liu, C.-J. Molecules 2019, 24, 3838. |
| [13] | Denis, P.; Andy, W.; John, H.; Sneddon, H.; C. Robert, M.; Sarah, A. S.; Peter J. D. Green Chem. 2016, 18, 288. |
| [14] | Zhao, C.-Q.; Chen, Y.-G.; Qiu, H.; Wei, L.; Fang, P.; Mei, T.-S. Org. Lett. 2019, 21, 1412. |
| [15] | Stein, A.-L.; Bilheria, F.-N.; Zeni, G. Chem. Commun. 2015, 51, 15522. |
| [16] | (a) Qiu, S.-Z.; Zhang, C.-Y.; Qiu, R.; Yin, G.-D.; Huang, J.-K. Adv. Synth. Catal. 2018, 360, 313. |
| [16] | (b) Yu, S.-Y.; Wu, J.-X.; He, X.-W.; Shang, Y.-J. Appl. Organomet. Chem. 2018, 32, e4156. |
| [16] | (c) Topolov?an, N.; Hara, S.; Císa?ová, I.; To?ner, Z.; Kotora, M. Eur. J. Org. Chem. 2020, 2, 234. |
| [16] | (d) Jitendra, R. H.; Theodore, J. A.; Alwyn, T. G.; Garcia, M. T.; Robert, D. S.; Peter, J. S. Green Chem. 2010, 12, 650. |
| [16] | (e) Pati, A. K.; Mohapatra, M.; Ghosh, P.; Gharpure, S. J.; Mishra, A. K. J. Phys. Chem. A 2013, 117, 6548. |
| [16] | (f) Kurita, T.; Abe, M.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Synlett 2007, 16, 2521. |
| [16] | (g) Chen, S.-N.; Wu, W.-Y.; Tsai, F.-Y. Green Chem. 2009, 11, 269. |
| [16] | (h) Batsanov, A. S.; Collings, J. C.; Fairlamb, I. J. S.; Holland, J. P.; Howard, J. A. K.; Lin, Z.-Y.; Marder, T. B.; Parsons, A. C.; Ward, R. M.; Zhu, J. J. Org. Chem. 2005, 70, 703. |
| [16] | (i) Li, X.; Li, D.-J.; Bai, Y.-N.; Zhang, C.-X.; Chang, H.-H.; Gao, W.-C.; Wei, W.-L. Tetrahedron 2016, 72, 6996. |
| [16] | (j) Zhang, S.-L.; Liu, X.-Y.; Wang, T.-Q. Adv. Synth. Catal. 2011, 353, 1463. |
| [16] | (k) Chinta, B. S.; Baire, B. RSC Adv. 2016, 6, 54449. |
/
| 〈 |
|
〉 |