

水溶液中的Minisci反应研究进展
收稿日期: 2024-01-15
修回日期: 2024-03-05
网络出版日期: 2024-03-28
基金资助
国家自然科学基金(31972850)
Recent Advances of Minisci Reactions in Aqueous Solution
Received date: 2024-01-15
Revised date: 2024-03-05
Online published: 2024-03-28
Supported by
National Natural Science Foundation of China(31972850)
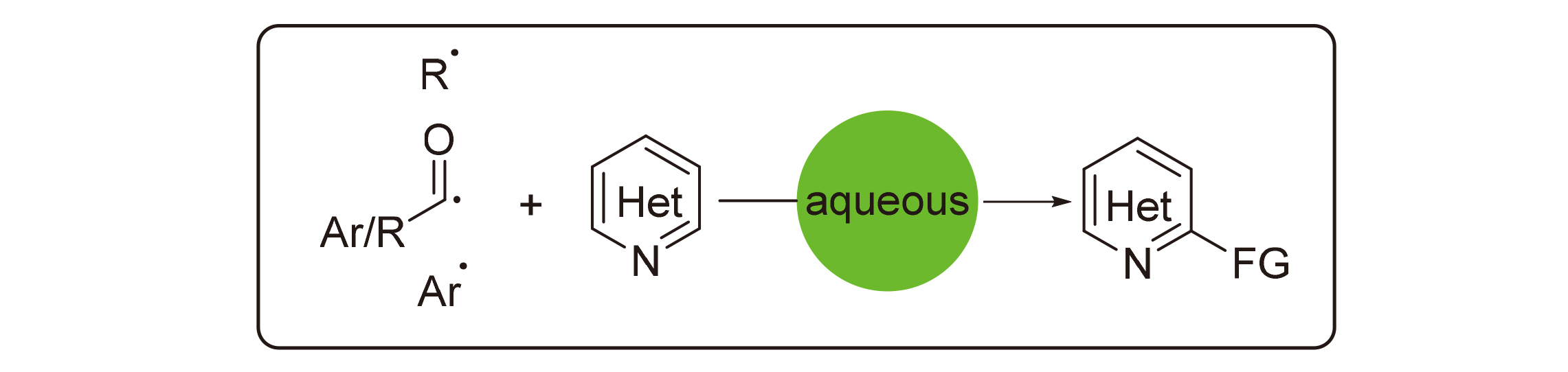
Minisci反应是通过自由基中间体对缺电子含氮杂环化合物进行官能团化的方法. 利用Minisci反应可以向未官能团化的缺电子含氮杂环上引入烷基、酰基、硅基、烷硫基、硼烷等, 构建具有不同取代基的杂环化合物, 是合成取代杂环的一个重要手段. 水作为一种绿色环保的溶剂, 是进行有机反应的理想介质, 在Minisci反应中得到了广泛应用. 尽管从发现Minisci反应开始, 水作为Minisci反应的介质就得到应用, 但是水溶液中的Minisci反应仍然发展缓慢. 从烷基化、芳基化和酰基化三个方面, 根据不同的自由基前体, 综述了水溶液中Minisci反应的进展.
王文贵 , 王守锋 . 水溶液中的Minisci反应研究进展[J]. 有机化学, 2024 , 44(7) : 2136 -2146 . DOI: 10.6023/cjoc202401015
Minisci reaction is a convenient method for the functionalization of C—H in electron-defficient N-heterocycles. Different substituents could be introduced onto heterocycles through Minisci reaction, such as alkyl, acyl, silyl and alkylthio, etc. Water is eco-friendly and an ideal medium in organic synthesis, which has been applied in Minisci reaction. Recent progress in Minisci reaction in aqueous solution is reviewed herein. Minisci reaction is a convenient method for the functionalization of electron-defficient N-heterocycles. Different substituents could be introduced onto heterocycles through Minisci reaction, such as alkyl, acyl, silyl and alkylthio, boryl, etc. Thus, Minisci reaction is a powerful toolbox for the synthesis of substituted N-heterocylces. Water is eco-friendly and an ideal medium in organic synthesis, which has been applied in Minisci reaction. Although water was used from the discovery of Minisci reaction, Minisci reaction in aqueous solution is still underdeveloped. The recent progress in Minisci reaction in aqueous solution, including alkylation, arylation and acylation, is reviewed according to different radical precursors.

Key words: Minisci reaction; radical; aqueous solution; heterocycle
| [1] | For selected reviews, see: (a) Liu, C.-X.; Yin, S.-Y.; Zhao, F.; Yang, H.; Feng, Z.; Gu, Q.; You, S.-L. Chem. Rev. 2023, 123, 10079. |
| [1] | (b) Zhang, J.; Rueping, M. Chem. Soc. Rev. 2023, 52, 4099. |
| [1] | (c) Josephitis, C. M.; Nguyen, H. M. H.; McNally, A. Chem. Rev. 2023, 123, 7655. |
| [1] | (d) Roy, S.; Panja, S.; Sahoo, S. R.; Chatterjee, S.; Maiti, D. Chem. Soc. Rev. 2023, 52, 2391. |
| [1] | (e) Bellotti, P.; Huang, H.-M.; Faber, T.; Glorius, F. Chem. Rev. 2023, 123, 4237. |
| [2] | Lu, M.-Z.; Goh, J.; Maraswami, M.; Jia, Z.; Tian, J.-S.; Loh, T.-P. Chem. Rev. 2022, 122, 17479. |
| [3] | For selected reviews and articles, see: (a) Baco?, P. D.; Lahdenper?, A. S. K.; Phipps, R. J. Acc. Chem. Res. 2023, 56, 2037. |
| [3] | (b) Dong, J.; Liu, Y.; Wang, Q. Chin. J. Org. Chem. 2021, 41, 3771. (in Chinese) |
| [3] | (董建洋, 刘玉秀, 汪清民, 有机化学, 2021, 41, 3771.) |
| [3] | (c) Meng, W.; Xu, K.; Guo, B.; Zeng, C. Chin. J. Org. Chem. 2021, 41, 2621. (in Chinese) |
| [3] | (孟薇, 徐坤, 郭兵兵, 曾程初, 有机化学, 2021, 41, 2621.) |
| [3] | (d) Wang, W.; Wang, S. Curr. Org. Chem. 2021, 25, 894. |
| [3] | (e) Zheng, H.; Lu, H.; Su, C.; Yang, R.; Zhao, L.; Liu, X.; Cao, H. Chin. J. Chem. 2023, 41, 193. |
| [4] | Minisci, F.; Vismara, E.; Fontana, F.; Morini, G.; Serravalle, M. J. Org. Chem. 1987, 52, 730 |
| [5] | Minisci, F. Tetrahedron 1971, 27, 3575. |
| [6] | Minisci, F.; Vismara, E. Tetrahedron Lett. 1986, 26, 4803. |
| [7] | Shore, D. G. M.; Wasik, K. A.; Lyssikatos, J. P.; Estrada, A. A. Tetrahedron Lett. 2015, 56, 4063. |
| [8] | Tung, T. T.; Christensen, S. B.; Nielsen, J. Chem.-Eur. J. 2017, 23, 18125. |
| [9] | Xie, X.; Zhang, Y.; Hao, J.; Wan, W. Org. Biomol. Chem. 2020, 18, 400. |
| [10] | Mai, D. N.; Baxter, R. D. Org. Lett. 2016, 18, 3738. |
| [11] | Galloway, J. D.; Mai, D. N.; Baxter, R. D. Org. Lett. 2017, 19, 5772. |
| [12] | Wang, W.; Song, Y.; Xing, S.; Li, J.; Feng, W.; Qu, X.; Wang, S. ChemistrySelect 2023, 8, e202300958. |
| [13] | Sutherland, D. R.; Veguillas, M.; Oates, C. L.; Lee, A.-L. Org. Lett. 2018, 20, 6863. |
| [14] | Jafarpour, F.; Darvishmolla, M.; Azaddoost, N.; Mohaghegh, F. New J. Chem. 2019, 43, 9328. |
| [15] | Dong, J.; Wang, Z.; Wang, X.; Song, H.; Liu, Y.; Wang, Q. J. Org. Chem. 2019, 84, 7532. |
| [16] | Shao, M.; Liang, H.; Liu, Y.-L.; Qin, W.; Li, Z. Asian J. Org. Chem. 2020, 9, 782. |
| [17] | Jin, J.; MacMillan, D. W. C. Angew. Chem., Int. Ed. 2015, 54, 1565. |
| [18] | Devariab, S.; Shah, B. A. Chem. Commun. 2016, 52, 1490. |
| [19] | McCallum, T.; McCallum, L.-A.; McCallum, A.; Barriault, L. Synlett 2016, 27, 1282. |
| [20] | Bohman, B.; Berntsson, B.; Dixon, R. C. M.; Stewart, C. D.; Barrow, R. A. Org. Lett. 2014, 16, 2787. |
| [21] | Xu, D.; Huang, F.; Tang, L.; Zhang, X.; Zhang, W. Chin. J. Org. Chem. 2022, 42, 1493. (in Chinese) |
| [21] | (徐东平, 黄飞, 汤琳, 张新明, 张武, 有机化学, 2022, 42, 1493.) |
| [22] | Lu, S.-C.; Li, H.-S.; Xu, S.; Duan, G.-Y. Org. Biomol. Chem. 2017, 15, 324. |
| [23] | Wu, X.; Wang, M.; Huan, L.; Wang, D.; Wang, J.; Zhu, C. Angew. Chem., Int. Ed. 2018, 57, 1640. |
| [24] | (a) Wang, Z.; Ji, X.; Zhao, J.; Huang, H. Green Chem. 2019, 21, 5512. |
| [24] | (b) Ji, X.; Liu, Q.; Wang, Z.; Wang, P.; Deng, G.-J.; Huang, H. Green Chem. 2020, 22, 8233. |
| [25] | Bosset, C.; Beucher, H.; Bretel, G.; Pasquier, E.; Queguiner, L.; Henry, C.; Vos, A.; Edwards, J. P.; Meerpoel, L.; Berthelot, D. Org. Lett. 2018, 20, 6003. |
| [26] | Santos, M. S.; Cybularczyk-Cecotka, M.; K?nig, B.; Giedyk, M. Chem. Eur. J. 2020, 26, 15323. |
| [27] | Molander, G. A.; Colombel, V.; Braz, V. A. Org. Lett. 2011, 13, 1852. |
| [28] | Presset, M.; Fleury-Brégeot, N.; Oehlrich, D.; Rombouts, F.; Molander, G. A. J. Org. Chem. 2013, 78, 4615. |
| [29] | Matsui, J. K.; Molander, G. A. Org. Lett. 2017, 19, 950. |
| [30] | Matsui, J. K.; Primer, D. N.; Molander, G. A. Chem. Sci. 2017, 8, 3512. |
| [31] | Yan, H.; Hou, Z.-W.; Xu, H.-C. Angew. Chem., Int. Ed. 2019, 58, 4592. |
| [32] | Gutie?rrez-Bonet, á.; Remeur, C.; Matsui, J. K.; Molander, G. A. J. Am. Chem. Soc. 2017, 139, 12251. |
| [33] | Ji, Y.; Brueckl, T.; Baxter, R. D.; Fujiwara, Y.; Seiple, I. B.; Su, S.; Blackmond, D. G.; Baran, P. S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 14411. |
| [34] | (a) Fujiwara, Y.; Dixon, J. A.; Rodriguez, R. A.; Baxter, R. D.; Dixon, D. D.; Collins, M. R.; Blackmond, D. G.; Baran, P. S. J. Am. Chem. Soc. 2012, 134, 1494. |
| [34] | (b) O’Hara, F.; Baxter, R. D.; O’Brien, A. G.; Collins, M. R.; Dixon, J. A.; Fujiwara, Y.; Ishihara, Y.; Baran, P. S. Nat. Protoc. 2013, 8, 1042. |
| [35] | Lytkinaa, M. A.; Eliseenkovb, E. V.; Boyarskiib, V. P.; Petrovb, A. A. Russ. J. Org. Chem. 2017, 53, 533. |
| [36] | Zhou, Q.; Ruffoni, A.; Gianatassio, R.; Fujiwara, Y.; Sella, E.; Shabat, D.; Baran, P. S. Angew. Chem., nt. Ed. 2013, 52, 3949. |
| [37] | Gianatassio, R.; Kawamura, S.; Eprile, C. L.; Foo, K.; Ge, J.; Burns, A. C.; Collins, M. R.; Baran, P. S. Angew. Chem., Int. Ed. 2014, 53, 9851. |
| [38] | Jia, X.-M.; Wei, L.; Chen, F.; Tang, R.-Y. RSC Adv. 2015, 5, 29766. |
| [39] | Seiple, I. B.; Su, S.; Rodriguez, R. A.; Gianatassio, R.; Fujiwara, Y.; Sobel, A. L.; Baran, P. S. J. Am. Chem. Soc. 2010, 132, 13194. |
| [40] | Patel, N. R.; Flowers, R. A. J. Am. Chem. Soc. 2013, 135, 4672. |
| [41] | Baxter, R. D.; Liang, Y.; Hong, X.; Brown, T. A.; Zare, R. N.; Houk, K. N.; Baran, P. S.; Blackmond, D. G. ACS Cent. Sci. 2015, 1, 456. |
| [42] | Biaco, J. L.; Jones, S. L.; Barker, T. J. Heterocycles 2016, 92, 1687. |
| [43] | Xue, D.; Jia, Z.-H.; Zhao, C.-J.; Zhang, Y.-Y.; Wang, C.; Xiao, J. Chem.-Eur. J. 2014, 20, 2960. |
| [44] | Wang, R.; Falck, J. R. Org. Chem. Front. 2014, 1, 1029. |
| [45] | Fontana, F.; Minisci, F.; Barbosa, M. C. N.; Vismara, E. J. Org. Chem. 1991, 56, 2866. |
| [46] | Zeng, X.; Liu, C.; Wang, X.; Zhang, J.; Wang, X.; Hu, Y. Org. Biomol. Chem. 2017, 15, 8929. |
| [47] | Regan, C. F.; Pierre, F.; Schwaebe, M. K.; Haddach, M.; Jung, M. E.; Ryckman, D. M. Synlett 2012, 23, 443. |
| [48] | Wang, X.-Z.; Zeng, C.-C. Tetrahedron 2019, 75, 1425. |
| [49] | Manna, S.; Prabhu, K. R. J. Org. Chem. 2019, 84, 5067. |
| [50] | Caronna, T.; Gardini, G. P.; Minisci, F. J. Chem. Soc. D 1969, 201. |
| [51] | (a) Caronna, T.; Fronza, G.; Minisci, F.; Porta, O. J. Chem. Soc., Perkin Trans. 2 1972, 2035. |
| [51] | (b) Minisci, F.; Citterio, A.; Vismara, E.; Giordano, C. Tetrahedron 1986, 41, 4157. |
| [52] | Song, Y.; Yu, Z.; Wang, W.; Wang, S. Tetrahedron 2023, 141, 133518. |
| [53] | Sultan, S.; Ahmad Rizvi, M. A.; Kumar, J.; Shah, B. A. Chem. Eur. J. 2018, 24, 10617. |
| [54] | Sharma, S.; Kumar, M.; Vishwakarma, R. A.; Verma, M. K.; Singh, P. P. J. Org. Chem. 2018, 83, 12420. |
/
| 〈 |
|
〉 |