

可见光促进活化烯烃叠氮化/环化反应合成吲哚[2,1-a]异喹啉酮衍生物研究
收稿日期: 2024-01-29
修回日期: 2024-03-19
网络出版日期: 2024-04-10
基金资助
湖南省教育厅科学研究基金(23B0650); 湖南省自然科学基金(2022JJ30418); 湖南文理学院重点研究项目(22ZD04); 大学生创新创业训练(XDC202318)
Visible-Light-Induced Cascade Azidation/Cyclization of Activated Alkenes to Synthesize Azidated Indolo[2,1-a]isoquinolines
Received date: 2024-01-29
Revised date: 2024-03-19
Online published: 2024-04-10
Supported by
Scientific Research Foundation of Hunan Provincial Education Department(23B0650); Natural Science Foundation of Hunan Province(2022JJ30418); Key Scientific Research Foundation of Hunan University of Arts and Science(22ZD04); Innovation and Entrepreneurship Training Program for College Students of Hunan University of Arts and Science(XDC202318)
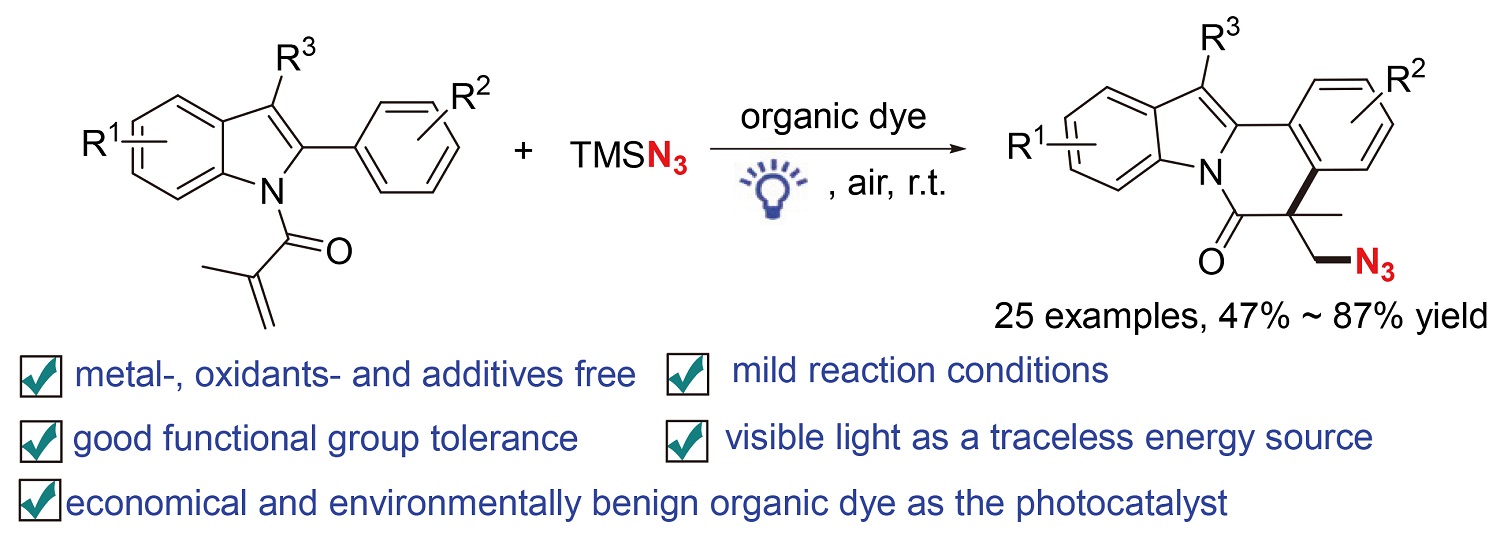
发展了一种以Rose Bengal为有机染料光催化剂, 在可见光照射下实现2-芳基吲哚与叠氮三甲基硅烷(TMSN3)的自由基叠氮化/环化反应构建叠氮基取代吲哚[2,1-a]异喹啉酮衍生物的方法. 该方法无需金属催化剂和添加剂, 以空气为氧化剂, 以中等到良好的收率得到结构多样的吲哚[2,1-a]异喹啉酮类化合物. 机理验证实验表明, 该反应经历了自由基历程.
关键词: Rose Bengal; 可见光; 2-芳基吲哚; 叠氮化; 吲哚[2,1-a]异喹啉酮
唐裕才 , 何宇鹏 , 杨碧玉 , 段京林 , 杜昌远 , 蒋洁 , 李佳丽 , 潘若涵 , 陈宇 , 刘学文 . 可见光促进活化烯烃叠氮化/环化反应合成吲哚[2,1-a]异喹啉酮衍生物研究[J]. 有机化学, 2024 , 44(7) : 2286 -2295 . DOI: 10.6023/cjoc202401034
An efficient visible-light-induced radical cascade azidation/cyclization of 2-aryl indoles with trimethylsilyl azide (TMSN3) has been developed using organic dye Rose Bengal as the photocatalyst. This method did not require metal catalysts and additives, and used air as the oxidant to obtain diverse indolo[2,1-a]isoquinolin-6(5H)-ones in moderate to good yields. Mechanistic studies demonstrated that the reaction proceeded via a radical pathway.

| [1] | (a) Br?se, S.; Gil, C.; Knepper, K.; Zimmermann, V. Angew. Chem., Int. Ed. 2005, 44, 5188. |
| [1] | (b) Br?se, S.; Banert, K. Organic Azides: Syntheses and Applications, Wiley, New York, 2010. |
| [1] | (c) Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Chem. Rev. 2013, 113, 4905. |
| [2] | Scriven, E. F. V.; Turnbull, K. Chem. Rev. 1988, 88, 297. |
| [3] | Kumar, R.; Wang, L. L.; Wiebe, L. I.; Knaus, E. E. J. Med. Chem. 1994, 37, 4297. |
| [4] | (a) Borreguero, A. M.; Mu?oz, M.; Haro, J. C. D.; Carmona, M.; Rodríguez, J. F. React. Funct. Polym. 2016, 101, 1. |
| [4] | (b) Vora, A.; Nasrullah, M. J.; Webster, D. C. J. Coat. Technol. Res. 2010, 7, 409. |
| [5] | (a) Patai, S. The Chemistry of the Azido Group, Wiley, London, 1971. |
| [5] | (b) Tron, G. C.; Pirali, T.; Billington, R. A.; Canonico, P. L.; Sorba, G.; Genazzani, A. A. Med. Res. Rev. 2008, 28, 278. |
| [5] | (c) Tanimoto, H.; Kakiuchi, K. Nat. Prod. Commun. 2013, 8, 1021. |
| [5] | (d) Lahabb, J. Click Chemistry for Biotechnology and Materials Science, Wiley, London, 2009. |
| [5] | (e) Gao, Y.; Zhang, X.; Yu, J.; Zhou, J. Acta Chim. Sinica 2023, 81, 1590. (in Chinese) |
| [5] | (高杨, 张学鑫, 余金生, 周剑, 化学学报, 2023, 81, 159.) |
| [6] | Sivaguru, P.; Ning, Y.; Bi, X. Chem. Rev. 2021, 121, 4253. |
| [7] | Matcha, K.; Narayan, R.; Antonchick, A. P. Angew. Chem., Int. Ed. 2013, 52, 7985. |
| [8] | Wang, J.-J.; Yu, W. Chem.-Eur. J. 2019, 25, 3510. |
| [9] | Ge, L.; Zhou, H.; Chiou, M.-F.; Jiang, H.; Jian, W.; Ye, C.; Li, X.; Zhu, X.; Xiong, H.; Li, Y.; Song, L.; Zhang, X.; Bao, H. Nat. Catal. 2021, 4, 28. |
| [10] | (a) Xie, J.; Jin, H.; Xu, P.; Zhu, C. Tetrahedron Lett. 2014, 55, 36. |
| [10] | (b) Reckenth?lera, M.; Griesbeck, A. G. Adv. Synth. Catal. 2013, 355, 2727. |
| [10] | (c) Xuan, J.; Lu, L.-Q.; Chen, J.-R.; Xiao, W.-J. Eur. J. Org. Chem. 2013, 2013, 6755. |
| [10] | (d) Zou, Y.-Q.; Chen, J.-R.; Xiao, W.-J. Angew. Chem., Int. Ed. 2013, 52, 11701. |
| [10] | (e) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322. |
| [10] | (f) Xi, Y.; Yi, H.; Lei, A. Org. Biomol. Chem. 2013, 11, 2387. |
| [10] | (g) Shang, T.-Y.; Lu, L.-H.; Cao, Z.; Liu, Y.; He, W.-M.; Yu, B. Chem. Commun. 2019, 55, 5408. |
| [10] | (h) Chen, J.-Y.; Wu, H.-Y.; Song, H.-Y.; Li, H.-X.; Yang, T.-B.; He, W.-M. J. Org. Chem. 2023, 88, 8360. |
| [10] | (i) Song, H.-Y.; Xiao, F.; Jiang, J.; Wu, C.; Ji, H.-T.; Lu, Y.-H.; Wang, K.-L.; He, W.-M. Chin. Chem. Lett. 2023, 34, 108509. |
| [10] | (j) Sun, Y-L.; Tan, F.-F.; Hu R.-G.; Hu C.-H.; Li Y. Chin. J. Chem., 2022, 40, 1903. |
| [11] | For recent examples using organic dyes as the photoredox catalyst see: (a) Rueping, M.; Vila, C.; Bootwicha, T. ACS Catal. 2013, 3, 1676. |
| [11] | (b) Hari, D. P.; Schroll, P.; K?nig, B. J. Am. Chem. Soc. 2012, 134, 2958. |
| [11] | (c) Hari, D. P.; Hering, T.; K?nig, B. Org. Lett. 2012, 14, 5334. |
| [11] | (d) Hering, T.; Hari, D. P.; K?nig, B. J. Org. Chem. 2012, 77, 10347. |
| [11] | (e) Liu, Q.; Li, Y.-N.; Zhang, H.-H.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Chem.-Eur. J. 2012, 18, 620. |
| [11] | (f) Fidaly, K.; Ceballos, C.; Falguières, A.; Veitia, M. S.-I.; Guy, A.; Ferroud, C. Green Chem. 2012, 14, 1293. |
| [12] | Zhou, N.; Xia, Z.; Wu, S.; Kuang, K.; Xu, Q.; Zhang, M. J. Org. Chem. 2021, 86, 15253. |
| [13] | Kulthe, A. D.; Jaiswal, S.; Golagani, D.; Mainkar, P. S.; Akondi, S. M. Org. Biomol. Chem. 2022, 20, 4534. |
| [14] | (a) Wei, W.; Cui, H. H.; Yue, H. L.; Yang, D. S. Green Chem. 2018, 20, 3197. |
| [14] | (b) Pei, C.; Liu, Y.; Chen, X.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Adv. Synth. Catal. 2023, 356, 860. |
| [14] | (c) Wu, X.; Zhang, X.; Ji, X.; Deng, G.-J.; Huang, H. Org. Lett. 2023, 25, 5162. |
| [15] | (a) Horton, D. A.; Bourne, G. T.; Smythe, M. L. Chem. Rev, 2003, 103, 893. |
| [15] | (b) Kochanowska-Karamyan, A. J.; Hamann, M. T. Chem. Rev. 2010, 110, 4489. |
| [15] | (c) Somei, M.; Yamada, F. Nat. Prod. Rep. 2004, 21, 278. |
| [16] | (a) Li, H.-C.; Sun, K.; Li, X.; Wang, S.-Y.; Chen, X.-L.; He, S.-Q.; Qu, L.-B.; Yu, B. J. Org. Chem. 2021, 86, 9055. |
| [16] | (b) Pan, Y.; Gong, X.; Hao, R.; Zeng, S.; Xu, J.; Shen, Z.; Huang, W. Asian J. Org. Chem. 2022, 11, e202100766. |
| [16] | (c) Hu, X.-Y.; Xu, H.-F.; Chen, Q.; Pan, Y.-L.; Chen, J.-Z. Org. Biomol. Chem. 2021, 19, 10376. |
| [16] | (d) Wei, Y.-L.; Chen, J.-Q.; Sun, B.; Xu, P.-F. Chem. Commun. 2019, 55, 5922. |
| [16] | (e) Cui, H.; Ni, C.; Zhang, C. J. Org. Chem., 2021, 86, 15835. |
| [16] | (f) Luo, Y.; Tian, T.; Nishihara, Y.; Lv, L.; Li, Z. Chem. Commun. 2021, 57, 9276. |
| [16] | (g) Zhang, J.-R.; Liu, H.-Y.; Fan, T.; Chen, Y.-Y.; Xu, Y.-L. Adv. Synth. Catal. 2021, 363, 497. |
| [17] | (a) Maiti, D.; Mahanty, K.; Sarkar, S. D. Chem.-Asian J. 2021, 16, 748. |
| [17] | (b) Srivastava, A.; Singh, P. K.; Ali, A.; Singh, P. P.; Srivastava, V. RSC Adv. 2020, 10, 39495. |
| [18] | Chen, Y.-X.; Wang, Z.-J.; Xiao, J.-A.; Chen, K.; Xiang, H.-Y.; Yang, H. Org. Lett. 2021, 23, 6558. |
/
| 〈 |
|
〉 |