

基于烯烃和炔烃的加成反应合成有机锗化合物的研究进展
收稿日期: 2024-01-14
修回日期: 2024-03-18
网络出版日期: 2024-04-10
基金资助
国家自然科学基金(22201300); 国家自然科学基金(22071266); 中央高校基本科研业务费专项资金和中国人民大学科研业务费专项资金(21XNLG04)
Advances in the Synthesis of Organogermaniums Based on the Addition Reactions of Alkenes and Alkynes
Received date: 2024-01-14
Revised date: 2024-03-18
Online published: 2024-04-10
Supported by
National Natural Science Foundation of China(22201300); National Natural Science Foundation of China(22071266); Fundamental Research Funds for the Central Universities, and the Research Funds of Renmin University of China(21XNLG04)
吕雷阳 , 罗亚妮 , 李志平 . 基于烯烃和炔烃的加成反应合成有机锗化合物的研究进展[J]. 有机化学, 2024 , 44(7) : 2092 -2109 . DOI: 10.6023/cjoc202401014
Organogermanium compounds have a wide range of potential applications, especially in the fields of organic synthesis, medicinal chemistry and materials sciences. However, compared with the progress of other elements of the carbon family, the study and development of organogermanium chemistry have long been relatively scattered and sluggish, although it has attracted increasing attention from chemists in recent years. This review aims to introduce the research progress in the synthesis of organogermanium compounds via the addition reactions of alkenes and alkynes. The progress is classified according to the types of addition reactions of germanium with alkenes and alkynes, different reaction systems, reaction mechanisms and synthetic protocols. In addition, the problems encountered during the research process and future development trends are also prospected.
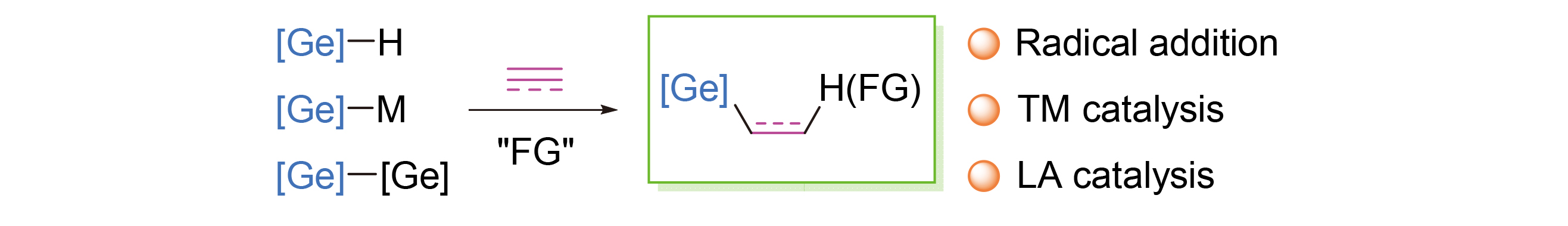
Key words: organogermanium; alkene; alkyne; hydrogermylation; addition reaction
| [1] | Winkler, C. J. Prakt. Chem. 1887, 36, 177. |
| [2] | (a) Buriak, J. M. Chem. Rev. 2002, 102, 1271. |
| [2] | (b) Lee, V. Y. Organogermanium Compounds Theory, Experiment, and Applications, John Wiley & Sons, Inc., New Jersey, 2023. |
| [3] | (a) Spivey, A. C.; Gripton, C. J. G.; Hannah, J. P. Curr. Org. Synth. 2004, 1, 211. |
| [3] | (b) Wang, H. Chin. Chem. Lett. 2022, 33, 3672. |
| [4] | Ma, K., Luo, S., Chen, C., Zhang, W., Wang, F. Conserv. Util. Miner. Resour. 2019, 39, 16. (in Chinese) |
| [4] | (马奎, 洛桑才仁, 陈超, 张万益, 王丰翔, 矿产保护与利用, 2019, 39, 16.) |
| [5] | (a) Weinert, C. S. In Encyclopedia of Inorganic and Bioinorganic Chemistry, Wiley, Chichester, 2015, pp. 1-18. |
| [5] | (b) Drake, J. E.; Siebert, C.; W?bke, B. . 1994. |
| [6] | (a) Yamakawa, T.; Kagechika, H.; Kawachi, E.; Hashimoto, Y.; Shudo, K. J. Med. Chem. 1990, 33, 1430. |
| [6] | (b) Fujii, S.; Miyajima, Y.; Masuno, H.; Kagechika, H. J. Med. Chem. 2013, 56, 160. |
| [7] | (a) Moon, M.; Walker, B.; Lee, J.; Park, S. Y.; Ahn, H.; Kim, T.; Lee, T. H.; Heo, J.; Seo, J. H.; Shin, T. J.; Kim, J. Y.; Yang, C. Adv. Energy Mater. 2015, 5, 1402044. |
| [7] | (b) Hung, M.-K.; Tsai, K.-W.; Sharma, S.; Wu, J.-Y.; Chen, S.-A. Angew. Chem., Int. Ed. 2019, 58, 11317. |
| [7] | (c) Cui, J. Chin. J. Org. Chem. 2018, 38, 2888. (in Chinese) |
| [7] | (崔晶晶, 有机化学, 2018, 38, 2888.) |
| [7] | (d) Xu, X.; Zhou, J.; Guo, L.; Wu, H.; Shi, J.; Li, D.; Luo, Y.; Meng, Q. Chin. Sci. Bull. 2021, 66, 2202. (in Chinese) |
| [7] | (徐啸, 周家正, 郭林宝, 吴会觉, 石将建, 李冬梅, 罗艳红, 孟庆波, 科学通报, 2021, 66, 2202.) |
| [8] | For selected examples, see: (a) Xu, M.-Y.; Jiang, W.-T.; Li, Y.; Xu, Q.-H.; Zhou, Q.-L.; Yang, S.; Xiao, B. J. Am. Chem. Soc. 2019, 141, 7582. |
| [8] | (b) Jiang, W.-T.; Xu, M.-Y.; Yang, S.; Xie, X.-Y.; Xiao, B. Angew. Chem., Int. Ed. 2020, 59, 204504. |
| [8] | (c) Wang, C.; Liu, Y.-C.; Xu, M.-Y.; Xiao, B. Org. Lett. 2021, 23, 4593. |
| [8] | (d) Xu, M.-Y.; Xiao, B. Chem. Commun. 2021, 57, 11764. |
| [8] | (e) Yang, S.; Jiang, W.-T.; Xiao, B. Chem. Commun. 2021, 57, 8143. |
| [8] | (f) Xu, Q.-H.; Wei, L.-P.; Xiao, B. Angew. Chem., Int. Ed. 2022, 61, e202115592. |
| [8] | (g) Xu, Q.-H.; Xiao, B. Chemistry 2020, 83, 610. (in Chinese) |
| [8] | (徐清浩, 肖斌, 化学通报, 2020, 83, 610.) |
| [8] | (h) Xu, Q.-H.; Wei, L.-P.; Zhang, Z.; Xiao, B. Acta Chim. Sinica 2022, 80, 428. (in Chinese) |
| [8] | (徐清浩, 魏立谱, 张震, 肖斌, 化学学报, 2022, 80, 428.) |
| [9] | (a) Dahiya, A.; Fricke, C.; Schoenebeck, F. J. Am. Chem. Soc. 2020, 142, 7754. |
| [9] | (b) Fricke, C.; Deckers, K.; Schoenebeck, F. Angew. Chem., Int. Ed. 2020, 59, 18717. |
| [9] | (c) Fricke, C.; Schoenebeck, F. Acc. Chem. Res. 2020, 53, 2715. |
| [9] | (d) Sherborne, G. J.; Gevondian, A. G.; Funes-Ardoiz, I.; Dahiya, A.; Fricke, C.; Schoenebeck, F. Angew. Chem., Int. Ed. 2020, 59, 15543. |
| [9] | (e) Dahiya, A.; Schoenebeck, F. ACS Catal. 2022, 12, 8048. |
| [9] | (f) Kreisel, T.; Mendel, M.; Queen, A. E.; Deckers, K.; Hupperich, D.; Riegger, J.; Fricke, C.; Schoenebeck, F. Angew. Chem., Int. Ed. 2022, 61, e202201475. |
| [9] | (g) Selmani, A.; Schoetz, M. D.; Queen, A. E.; Schoenebeck, F. ACS Catal. 2022, 12, 4833. |
| [10] | (a) Su, P.-F.; Wang, K.; Peng, X.; Pang, X.; Guo, P.; Shu, X.-Z. Angew. Chem., Int. Ed. 2021, 60, 26571. |
| [10] | (b) Guo, P.; Pang, X.; Wang, K.; Su, P.-F.; Pan, Q.-Q.; Han, G.-Y.; Shen, Q.; Zhao, Z.-Z.; Zhang, W.; Shu, X.-Z. Org. Lett. 2022, 24, 1802. |
| [10] | (c) Pang, X.; Su, P.-F.; Shu, X.-Z. Acc. Chem. Res. 2022, 55, 2491. |
| [11] | Dobrzyński, D.; Boguszewska-Czubara, A.; Sugimori, K. Environ. Geochem. Health 2018, 40, 1355. |
| [12] | Song, H.-J.; Jiang, W.-T.; Zhou, Q.-L.; Xu, M.-Y.; Xiao, B. ACS Catal. 2018, 8, 9287. |
| [13] | (a) Jiang, W. T. Ph.D. Dissertation, University of Science and Technology of China, Hefei, 2021. (in Chinese) |
| [13] | (江伟韬, 博士论文,中国科学技术大学, 合肥, 2021.) |
| [13] | (b) Yorimitsu, H.; Oshima, K. Inorg. Chem. Commun. 2005, 8, 131. |
| [13] | (c) Dobbs, A. P.; Chio, F. K. I. In Comprehensive Organic Synthesis, 2nd ed., Ed.: Knochel, P., Elsevier, Amsterdam, 2014, pp. 964-998. |
| [13] | (d) Saptal, V. B.; Wang, R.; Park, S. RSC Adv. 2020, 10, 43539. |
| [13] | (e) He, F.; Wu, J. Chin. J. Org. Chem. 2022, 42, 312. (in Chinese) |
| [13] | (何福生, 吴劼, 有机化学, 2022, 42, 312.) |
| [13] | (f) Rogova, T.; Ahrweiler, E.; Schoetz, M. D.; Schoenebeck, F. Angew. Chem., Int. Ed. 2023, e202314709. |
| [14] | Zhao, Z.; Zhang, F.; Wang, D.; Deng, L. Chin. J. Chem. 2023, 41, 3063. |
| [15] | Fischer, A. K.; West, R. C.; Rochow, E. G. J. Am. Chem. Soc. 1954, 76, 5878. |
| [16] | Fuchs, R.; Gilman, H. J. Org. Chem. 1957, 22, 1009. |
| [17] | Satgé, J.; Rivière, P. J. Organomet. Chem. 1969, 16, 71. |
| [18] | Tanaka, S.; Nakamura, T.; Yorimitsu, H.; Shinokubo, H.; Oshima, K. Org. Lett. 2000, 2, 1911. |
| [19] | Miura, K.; Ootsuka, K.; Hosomi, A. J. Organomet. Chem. 2007, 692, 514. |
| [20] | Xu, N.-X.; Li, B.-X.; Wang, C.; Uchiyama, M. Angew. Chem., Int. Ed. 2020, 59, 10639. |
| [21] | Queen, A. E.; Selmani, A.; Schoenebeck, F. Org. Lett. 2022, 24, 406. |
| [22] | (a) Lusztyuk, J.; Maillard, B.; Deycard, S.; Lindsay, D. A.; Ingold, K. U. J. Org. Chem. 1987, 52, 3509. |
| [22] | (b) Chatgilialoglu, C.; Ballestri, M.; Escudié, J.; Pailhous, I. Organometallics 1999, 18, 2395. |
| [23] | Luo, Y.; Tian, T.; Nishihara, Y.; Lv, L.; Li, Z. Chem. Commun. 2021, 57, 9276. |
| [24] | Luo, Y.; Xu, B.; Lv, L.; Li, Z. Org. Lett. 2022, 24, 2425. |
| [25] | Luo, Y.; Lv, L.; Li, Z. Org. Lett. 2022, 24, 8052. |
| [26] | Ke, J.; Liu, W.; Zhu, X.; Tan, X.; He, C. Angew. Chem., Int. Ed. 2021, 60, 8744. |
| [27] | (a) Chalk, A. J.; Harrod, J. F. J. Am. Chem. Soc. 1965, 87, 16. |
| [27] | (b) Chalk, A. J.; Harrod, J. F. J. Am. Chem. Soc. 1965, 87, 1133. |
| [28] | Trofimov, A.; Rubina, M.; Rubin, M.; Gevorgyan, V. J. Org. Chem. 2007, 72, 8910. |
| [29] | Greenhalgh, M. D.; Thomas, S. P. Chem. Commun. 2013, 49, 11230. |
| [30] | Wollenburg, M.; Bajohr, J.; Marchese, A. D.; Whyte, A.; Glorius, F.; Lautens, M. Org. Lett. 2020, 22, 3679. |
| [31] | Lin, W.; You, L.; Yuan, W.; He, C. ACS Catal. 2022, 12, 14592. |
| [32] | Louka, A.; Stratakis, M. Org. Lett. 2021, 23, 3599. |
| [33] | Gu, R.; Feng, X.; Bao, M.; Zhang, X. Nat. Commun. 2023, 14, 7669. |
| [34] | Qiao, Y.-W.; Zhou, B.-Q.; Huang, Y. Chem. Catal. 2023, 3, 100819. |
| [35] | Keess, S.; Oestreich, M. Org. Lett. 2017, 19, 1898. |
| [36] | Ichinose, Y.; Nozaki, K.; Wakamatsu, K.; Oshima, K.; Utimoto, K. Tetrahedron Lett. 1987, 28, 3709. |
| [37] | Nozaki, K.; Ichinose, Y.; Wakamatsu, K.; Oshima, K.; Utimoto, K. Bull. Chem. Soc. Jpn. 1990, 63, 2268. |
| [38] | Kinoshita, H.; Kakiya, H.; Oshima, K. Bull. Chem. Soc. Jpn. 2000, 73, 2159. |
| [39] | Bernardoni, S.; Lucarini, M.; Pedulli, G. F.; Valgimigli, L.; Gevorgyan, V.; Chatgilialoglu, C. J. Org. Chem. 1997, 62, 8009. |
| [40] | David-Quillot, F.; Thiéry, V.; Abarbri, M.; Thibonnet, J.; Besson, T.; Duchéne, A. Main Group Met. Chem. 2007, 30, 235. |
| [41] | Liang, Y.; Pitteloud, J.-P.; Wnuk, S. F. J. Org. Chem. 2013, 78, 5761. |
| [42] | de la Vega-Hernández, K.; Romain, E.; Coffinet, A.; Bijouard, K.; Gontard, G.; Chemla, F.; Ferreira, F.; Jackowski, O.; Perez-Luna, A. J. Am. Chem. Soc. 2018, 140, 17632. |
| [43] | Liang, H.; Ji, Y.-X.; Wang, R.-H.; Zhang, Z.-H.; Zhang, B. Org. Lett. 2019, 21, 2750. |
| [44] | Luo, Y.; Lv, L.; Li, Z. ChemCatChem 2023, 15, e202300467. |
| [45] | Ichinose, Y.; Oda, H.; Oshima, K.; Utimoto, K. Bull. Chem. Soc. Jpn. 1987, 60, 3468. |
| [46] | Torres, N. M.; Lavis, J. M.; Maleczka, R. E. Tetrahedron Lett. 2009, 50, 4407. |
| [47] | Kinoshita, H.; Nakamura, T.; Kakiya, H.; Shinokubo, H.; Matsubara, S.; Oshima, K. Org. Lett. 2001, 3, 2521. |
| [48] | Radzhabov, M. R.; Mankad, N. P. Org. Lett. 2021, 23, 3221. |
| [49] | Bo?t, M.; Mermela, A.; ?ak, P. Chem. Commun. 2023, 59, 11548. |
| [50] | Schwier, T.; Gevorgyan, V. Org. Lett. 2005, 7, 5191. |
| [51] | de la Vega-Hernández, K.; Chemla, F.; Ferreira, F.; Jackowski, O.; Perez-Luna, A. Org. Lett. 2021, 23, 4426. |
| [52] | Matsuda, T.; Kadowaki, S.; Yamaguchi, Y.; Murakami, M. Org. Lett. 2010, 12, 1056. |
| [53] | Rummelt, S. M.; Radkowski, K.; Ro?ca, D.-A.; Fürstner, A. J. Am. Chem. Soc. 2015, 137, 5506. |
| [54] | Schweizer, S.; Tresse, C.; Bisseret, P.; Lalevée, J.; Evano, G.; Blanchard, N. Org. Lett. 2015, 17, 1794. |
| [55] | Debrauwer, V.; Turlik, A.; Rummler, L.; Prescimone, A.; Blanchard, N.; Houk, K. N.; Bizet, V. J. Am. Chem. Soc. 2020, 142, 11153. |
| [56] | Itazaki, M.; Kamitani, M.; Nakazawa, H. Chem. Commun. 2011, 47, 7854. |
| [57] | Sahoo, M. K.; Kim, D.; Chang, S.; Park, J.-W. ACS Catal. 2021, 11, 12777. |
| [58] | Zaranek, M.; Nowicki, M.; Andruszak, P.; Hoffmann, M.; Pawlu?, P. Chem. Commun. 2022, 58, 13979. |
| [59] | Dong, Z.; Albers, L.; Müller, T. Acc. Chem. Res. 2020, 53, 532. |
| [60] | Kassamba, S.; Perez-Luna, A.; Ferreira, F.; Durandetti, M. Chem. Commun. 2022, 58, 3901. |
| [61] | Kassamba, S.; Reboli, M.; Perez-Luna, A.; Ferreira, F.; Durandetti, M. Org. Chem. Front. 2023, 10, 3328. |
| [62] | Kojima, K.; Uchida, S.; Kinoshita, H.; Miura, K. Org. Lett. 2021, 23, 4598. |
| [63] | Xiang, X.; Zhou, Z.; Wu, X.; Ni, Z.; Gai, L.; Xiao, X.; Xu, L.; Zhao, Z.; Lu, H.; Guo, Z. CCS Chem. 2022, 4, 3798. |
/
| 〈 |
|
〉 |