

叔丁醇锂催化N-苄基-N-叔丁氧羰基酰胺与糖的酯化反应
收稿日期: 2024-01-30
修回日期: 2024-04-07
网络出版日期: 2024-04-30
基金资助
宁波工程学院启动基金(2130011540027)
t-BuOLi Catalyzed Esterification of N-Benzyl-N-Boc-amides with Carbohydrates
Received date: 2024-01-30
Revised date: 2024-04-07
Online published: 2024-04-30
Supported by
Startup Foundation of Ningbo University of Technology(2130011540027)
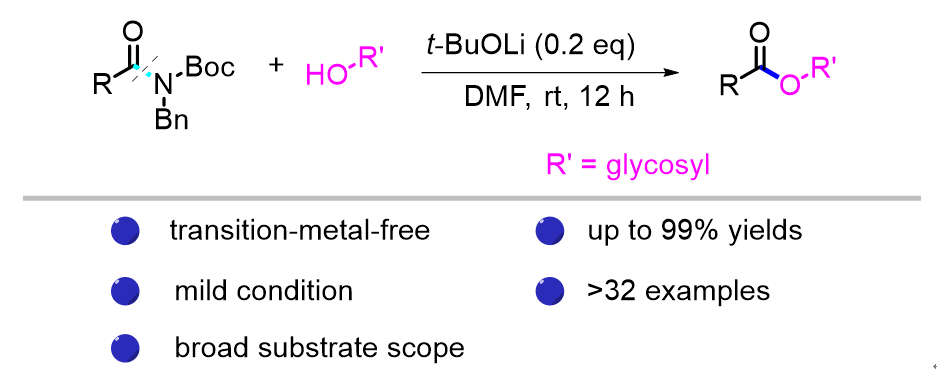
基于N—C键基态不稳定的策略, 报道了一种通过活化酰胺与糖类化合物发生酯化反应合成糖缀合物的新方法. 该方法采用温和的反应条件, 获得了优异的产率, 并具有广泛的底物兼容性, 展示了在糖缀合物合成中的巨大潜力. 此外, 该方法为解决糖类化合物中大空间位阻的次级羟基酯化这一具有挑战性的问题提供了一种替代途径, 并对糖类药物的发展具有促进作用.
关键词: 叔丁醇锂; N-benzyl-N-Boc酰胺; 酯化; 糖缀合物
叶丹锋 , 徐冰 , 万云辉 . 叔丁醇锂催化N-苄基-N-叔丁氧羰基酰胺与糖的酯化反应[J]. 有机化学, 2024 , 44(9) : 2924 -2932 . DOI: 10.6023/cjoc202401037
Based on a strategy of ground-state destabilisation of the N—C bond, a general protocol for the esterification of amides with carbohydrates was reported. This protocol offers mild reaction conditions, excellent yields, and broad substrate compatibility, thereby demonstrating great potential for the synthesis of glycoconjugates. Additionally, this method provides an alternative approach for addressing the challenging task of esterifying sterically hindered secondary hydroxyl group of carbohydrates and paving the way for advancements in carbohydrate-based pharmaceuticals.

Key words: t-BuOLi; N-benzyl-N-Boc-amides; esterification; glycoconjugates
| [1] | (a) Huang, M.; Li, J.-J.; Zhang, C. Green Chem. 2023, 25, 9187. |
| [1] | (b) Liu, J.; Parker, M. F. L.; Wang, S.; Flavell, R. R.; Toste, F. D.; Wilson, D. M. Chem 2021, 7, 2245. |
| [1] | (c) Meng, G.; Zhang, J.; Szostak, M. Chem. Rev. 2021, 121, 12746. |
| [1] | (d) Zeng, L.; Xu, S.; Cui, S.; Zhang, F. Org. Chem. Front. 2022, 9, 3757. |
| [2] | (a) Glatzhofer, D. T.; Roy, R. R.; Cossey, K. N. Org. Lett. 2002, 4, 2349. |
| [2] | (b) Toyao, T.; Nurnobi Rashed, M.; Morita, Y.; Kamachi, T.; Hakim Siddiki, S. M. A.; Ali, M. A.; Touchy, A. S.; Kon, K.; Maeno, Z.; Yoshizawa, K.; Shimizu, K.-I. ChemCatChem 2019, 11, 449. |
| [3] | (a) Hie, L.; Fine Nathel, N. F.; Shah, T. K.; Baker, E. L.; Hong, X.; Yang, Y.-F.; Liu, P.; Houk, K. N.; Garg, N. K. Nature 2015, 524, 79. |
| [3] | (b) Hie, L.; Baker, E. L.; Anthony, S. M.; Desrosiers, J.-N.; Senanayake, C.; Garg, N. K. Angew. Chem., Int. Ed. 2016, 55, 15129. |
| [4] | Bourne-Branchu, Y.; Gosmini, C.; Danoun, G. Chem.-Eur. J. 2017, 23, 10043. |
| [5] | Rahman, M. M.; Li, G.; Szostak, M. J. Org. Chem. 2019, 84, 12091. |
| [6] | Wu, H.; Guo, W.; Daniel, S.; Li, Y.; Liu, C.; Zeng, Z. Chem.-Eur. J. 2018, 24, 3444. |
| [7] | (a) Huang, C.; Li, J.; Wang, J.; Zheng, Q.; Li, Z.; Tu, T. Sci. China: Chem. 2021, 64, 66. |
| [7] | (b) Ding, H.; Qi, W.-Y.; Zhen, J.-S.; Ding, Q.; Luo, Y. Tetrahedron Lett. 2020, 61, 152444. |
| [8] | (a) La Ferla, B.; Airoldi, C.; Zona, C.; Orsato, A.; Cardona, F.; Merlo, S.; Sironi, E.; D'Orazio, G.; Nicotra, F. Nat. Prod. Rep. 2011, 28, 630. |
| [8] | (b) Davis, A. P. Chem. Soc. Rev. 2020, 49, 2531. |
| [9] | (a) Xu, J.; Du, H.; Shi, H.; Song, J.; Yu, J.; Zhou, Y. J. Exp. Bot. 2023, 74, 6119. |
| [9] | (b) Bissette, A. J. Commun. Chem. 2020, 3, 115. |
| [10] | (a) Lv, Z.; Liu, H.; Hao, H.; Rahman, F.-U.; Zhang, Y. Eur. J. Med. Chem. 2023, 249, 115164. |
| [10] | (b) Liet, B.; Laigre, E.; Goyard, D.; Todaro, B.; Tiertant, C.; Boturyn, D.; Berthet, N.; Renaudet, O. Chem. Eur. J. 2019, 25, 15429. |
| [10] | (c) Shi, B.; Chen, Y.; Geng, M. ; Zhu, D.; Yu, B. Chin. J. Chem. 2023, 41, 891. |
| [11] | (a) Apicella, M. Front. Cell. Infect. Microbiol. 2012, 2, 1. |
| [11] | (b) Zhao, M.; Zhu, Y.; Wang, H.; Zhang, W.; Mu, W. Synth. Syst. Biotechnol. 2023, 8, 509. |
| [12] | Kubo, S.; Mimaki, Y.; Terao, M.; Sashida, Y.; Nikaido, T.; Ohmoto, T. Phytochemistry 1992, 31, 3969. |
| [13] | (a) Morzycki, J. W.; Wojtkielewicz, A. Phytochem. Rev. 2005, 4, 259. |
| [13] | (b) Tang, Y.; Li, N.; Duan, J.-A.; Tao, W. Chem. Rev. 2013, 113, 5480. |
| [14] | (a) Walther, R.; Zelikin, A. N. Adv. Drug Delivery Rev. 2021, 171, 62. |
| [14] | (b) Zhang, P.; Woen, S.; Wang, T.; Liau, B.; Zhao, S.; Chen, C.; Yang, Y.; Song, Z.; Wormald, M. R.; Yu, C.; Rudd, P. M. Drug Discovery Today 2016, 21, 740. |
| [15] | (a) Meng, G.; Szostak, M. Org. Lett. 2015, 17, 4364. |
| [15] | (b) Meng, G., Szostak, M. Org. Biomol. Chem. 2016, 14, 5690. |
| [16] | (a) Ye, D.; Liu, Z.; Chen, H.; Sessler, J. L.; Lei, C. Org. Lett. 2019, 21, 6888. |
| [16] | (b) Ye, D.; Chen, H.; Liu, Z.; Lei, C. Chin. J. Org. Chem. 2021, 41, 1658 (in Chinese). |
| [16] | (叶丹锋, 陈浩, 刘志园, 雷川虎, 有机化学, 2021, 41, 1658.) |
/
| 〈 |
|
〉 |