

廉价金属配合物应用于均相催化酯加氢制醇的研究进展
收稿日期: 2024-03-19
修回日期: 2024-05-07
网络出版日期: 2024-05-30
基金资助
国家自然科学基金(21802010); 安徽省自然科学基金(2308085MB49); 安徽省优秀青年基金(2023AH030038); 安徽省优秀青年基金(gxyq2022027); 湖南省教育厅重点项目(23A0089); 湖南师范大学测试基金(23CSY016)
Advances in Homogeneous Hydrogenation of Esters to Alcohols by Non-noble Metal Complexes
Received date: 2024-03-19
Revised date: 2024-05-07
Online published: 2024-05-30
Supported by
National Natural Science Foundation of China(21802010); Anhui Provincial Natural Science Foundation(2308085MB49); Anhui Provincial Fund for Outstanding Young Scholars(2023AH030038); Anhui Provincial Fund for Outstanding Young Scholars(gxyq2022027); Key Program of Hunan Education Committee(23A0089); Measurement Funding of Hunan Normal University(23CSY016)
方霄龙 , 张钰 , 王韬 , 李斌 , 段宁 , 张峰君 . 廉价金属配合物应用于均相催化酯加氢制醇的研究进展[J]. 有机化学, 2024 , 44(11) : 3335 -3344 . DOI: 10.6023/cjoc202403024
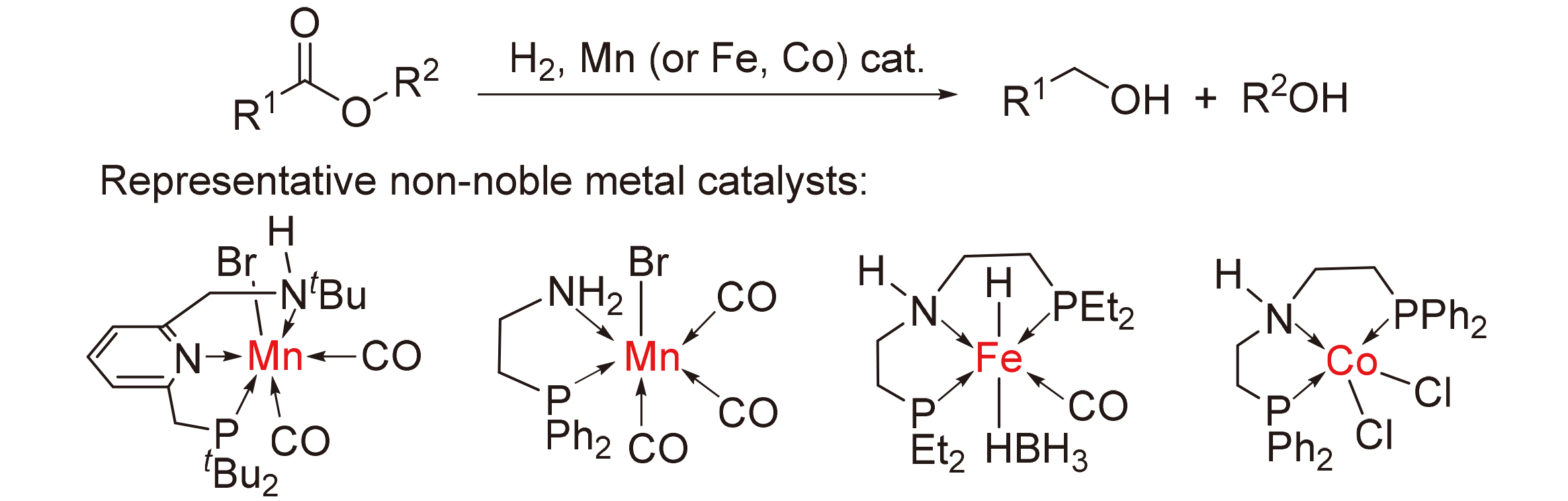
Catalytic hydrogenation of esters to alcohols is a green, sustainable, and atom-economic methodology for alcohol perparation. In the last two decades, the application of noble metal complexes (Ru(II), Ir(III), Os(II), etc.) in homogeneous catalytic hydrogenation of esters to alcohols has achieved significant progress. Replacing noble metals with abundant and environmentally friendly, non-noble metals has been becoming a hot spot. The progress in recent years on the homogeneous catalytic hydrogenation of esters to alcohols is summarized based on non-noble metal complexes including Fe(II), Co(II), and Mn(I). The relationship between complex structure and performance, as well as the catalytic hydrogenation reaction mechanism is detailed discussed to find out the key factors affecting the performance of the complexes, which provides new ideas for further design and development of excellent non-noble metal catalysts.
| [1] | (a) Dub P. A.; Ikariya T. ACS Catal. 2012, 2, 1718. |
| [1] | (b) Clarke M. L. Catal. Sci. Technol. 2012, 2, 2418. |
| [1] | (c) Li W.; Xie J.-H.; Yuan M. L.; Zhou Q. L. Green. Chem. 2014, 16, 4081. |
| [1] | (d) Gu X.; Li X.; Xie J.; Zhou Q. Acta Chim. Sinica 2019, 77, 598 (in Chinese). |
| [1] | (顾雪松, 李校根, 谢建华, 周其林, 化学学报, 2019, 77, 598.) |
| [2] | Ohkuma T.; Ooka H.; Ikariya T.; Noyori R. J. Am. Chem. Soc. 1995, 117, 10417. |
| [3] | (a) Noyori R.; Ohkuma T. Angew. Chem., Int. Ed. 2001, 40, 40. |
| [3] | (b) Noyori R. Angew. Chem., Int. Ed. 2002, 41, 2008. |
| [3] | (c) Sandoval C. A.; Ohkuma T.; Mu?iz K.; Noyori R. J. Am. Chem. Soc. 2003, 125, 13490. |
| [3] | (d) Zhao B.; Han Z.; Ding K. Angew. Chem., Int. Ed. 2013, 52, 4744. |
| [3] | (e) Werkmeister S.; Junge K.; Beller M. Org. Process Res. Dev. 2014, 18, 289. |
| [4] | Zhang J.; Leitus G.; Ben-David Y.; Milstein D. Angew. Chem., Int. Ed. 2006, 45, 1113. |
| [5] | Saudan L. A.; Saudan C. M.; Debieux C.; Wyss P. Angew. Chem., Int. Ed. 2007, 46, 7473. |
| [6] | Kuriyama W.; Matsumoto T.; Ogata O.; Ino Y.; Aoki K.; Tanaka S.; Ishida K.; Kobayashi T.; Sayo N.; Saito T. Org. Process Res. Dev. 2012, 16, 166. |
| [7] | (a) Sun Y.; Koehler C.; Tan R.; Annibale V. T.; Song D. Chem. Commun. 2011, 47, 8349. |
| [7] | (b) Spasyuk D.; Gusev D. G. Organometallics 2012, 31, 5239. |
| [7] | (c) Spasyuk D.; Smith S.; Gusev D. G. Angew. Chem., Int. Ed. 2012, 51, 2772. |
| [7] | (d) Yue H.; Zhao Y.; Ma X.; Gong J. Chem. Soc. Rev. 2012, 41, 4218. |
| [7] | (e) Chen T.; Li H.; Qu S.; Zheng B.; He L.; Lai Z.; Wang Z. X.; Huang K. W. Organometallics 2014, 33, 4152. |
| [7] | (f) Spasyuk D.; Vicent C.; Gusev D. G. J. Am. Chem. Soc. 2015, 137, 3743. |
| [7] | (g) Tan X.; Wang Y.; Liu Y.; Wang F.; Shi L.; Lee K.-H.; Lin Z.; Lv H.; Zhang X. Org. Lett. 2015, 17, 454. |
| [7] | (h) Wang F.; Tan X.; Lv H.; Zhang X. Chem.-Asian J. 2016, 11, 2103. |
| [8] | (a) Clarke Z. E.; Maragh P. T.; Dasgupta T. P.; Gusev D. G.; Lough A. J.; Abdur-Rashid K. Organometallics 2006, 25, 4113. |
| [8] | (b) Bertoli M.; Choualeb A.; Lough A. J.; Moore B.; Spasyuk D.; Gusev D. G. Organometallics 2011, 30, 3479. |
| [8] | (c) Acosta-Ramirez A.; Bertoli M.; Gusev D. G.; Schlaf M. Green Chem. 2012, 14, 1178. |
| [8] | (d) Otsuka T.; Ishii A.; Dub P. A.; Ikariya T. J. Am. Chem. Soc. 2013, 135, 9600. |
| [8] | (e) Junge K.; Wendt B.; Jiao H.; Beller M. ChemCatChem 2014, 6, 2810. |
| [8] | (f) Ogata O.; Nakayama Y.; Nara H.; Fujiwhara M.; Kayaki Y. Org. Lett. 2016, 18, 3894. |
| [9] | Werkmeister S.; Junge K.; Wendt B.; Alberico E.; Jiao H.; Baumann W.; Junge H.; Gallou F.; Beller M. Angew. Chem., Int. Ed. 2014, 53, 8722. |
| [10] | (a) Chakraborty S.; Dai H.; Bhattacharya P.; Fairweather N. T.; Gibson M. S.; Krause J. A.; Guan H. J. Am. Chem. Soc. 2014, 136, 7869. |
| [10] | (b) Qu S.; Dai H.; Dang Y.; Song C.; Wang Z. X.; Guan H. ACS Catal. 2014, 4, 4377. |
| [11] | Elangovan S.; Wendt B.; Topf C.; Bachmann S.; Scalone M.; Spannenberg A.; Jiao H.; Baumann W.; Junge K.; Beller M. Adv. Synth. Catal. 2016, 358, 820. |
| [12] | Elangovan S.; Garbe M.; Jiao H.; Spannenberg A.; Junge K.; Beller M. Angew. Chem., Int. Ed. 2016, 55, 15364. |
| [13] | Yuwen J.; Chakraborty S.; Brennessel W. W.; Jones W. D. ACS Catal. 2017, 7, 3735. |
| [14] | John J. M.; Takebayashi S.; Dabral N.; Miskolzie M.; Bergens S. H. J. Am. Chem. Soc. 2013, 135, 8578. |
| [15] | Junge K.; Wendt B.; Cingolani A.; Spannenberg A.; Wei Z.; Jiao H.; Beller M. Chem.-Eur. J. 2018, 24, 1046. |
| [16] | (a) Zhang G.; Hanson S. K. Org. Lett. 2013, 15, 650. |
| [16] | (b) R?sler S.; Ertl M.; Irrgang T.; Kempe R. Angew. Chem., Int. Ed. 2015, 54, 15046. |
| [16] | (c) Zhang G.; Yin Z.; Zheng S. Org. Lett. 2016, 18, 300. |
| [16] | (d) Mastalir M.; Tomsu G.; Pittenauer E.; Allmaier G.; Kirchner K. Org. Lett. 2016, 18, 3462. |
| [17] | Zhong R.; Wei Z.; Zhang W.; Liu S.; Liu Q. Chem 2019, 5, 1552. |
| [18] | Yang W.; Chernyshov I. Y.; van Schendel R. K.; Weber M.; Müller C.; Filonenko G. A.; Pidko E. A. Nat. Commun. 2021, 12, 12. |
| [19] | Yang W.; Kalavalapalli T. Y.; Krieger A. M.; Khvorost T. A.; Chernyshov I. Y.; Weber M.; Uslamin E. A.; Pidko E. A.; Filonenko G. A. J. Am. Chem. Soc. 2022, 144, 8129. |
| [20] | Wei Z.; Li H.; Wang Y.; Liu Q. Angew. Chem., Int. Ed. 2023, 62, e202301042. |
| [21] | (a) Gunanathan C.; Ben-David Y.; Milstein D. Science 2007, 317, 790. |
| [21] | (b) Gnanaprakasam B.; Zhang J.; Milstein D. Angew. Chem., Int. Ed. 2010, 49, 1468. |
| [21] | (c) Gunanathan C.; Gnanaprakasam B.; Iron M. A.; Shimon L. J.; Milstein D. J. Am. Chem. Soc. 2010, 132, 14763. |
| [21] | (d) Balaraman E.; Gunanathan C.; Zhang J.; Shimon L. J.; Milstein D. Nat. Chem. 2011, 3, 609. |
| [22] | Zell T.; Ben‐David Y.; Milstein D. Angew. Chem., Int. Ed. 2014, 53, 4685. |
| [23] | Srimani D.; Mukherjee A.; Goldberg A. F.; Leitus G.; Diskin‐ Posner Y.; Shimon L. J.; Ben David Y.; Milstein D. Angew. Chem., Int. Ed. 2015, 54, 12357. |
| [24] | Espinosa‐Jalapa N. A.; Nerush A.; Shimon L. J.; Leitus G.; Avram L.; Ben‐David Y.; Milstein D. Chem.-Eur. J. 2017, 23, 5934. |
| [25] | (a) Widegren M. B.; Harkness G. J.; Slawin A. M.; Cordes D. B.; Clarke M. L. Angew. Chem., Int. Ed. 2017, 56, 5825. |
| [25] | (b) Widegren M. B.; Clarke M. L. Org. Lett. 2018, 20, 2654. |
| [26] | Li X. G.; Li F.; Xu Y.; Xiao L. J.; Xie J. H.; Zhou Q. L. Adv. Synth. Catal. 2022, 364, 744. |
| [27] | Zubar V.; Lichtenberger N.; Schelwies M.; Oeser T.; Hashmi A. S. K.; Schaub T. ChemCatChem 2022, 14, e202101443. |
| [28] | Teunissen H. T.; Elsevier C. J. Chem. Commun. 1997, 667. |
| [29] | (a) Hanton M. J.; Tin S.; Boardman B. J.; Miller P. J. Mol. Catal. A: Chem. 2011, 346, 70. |
| [29] | (b) Geilen F. M.; Engendahl B.; Ho?lscher M.; Klankermayer J.; Leitner W. J. Am. Chem. Soc. 2011, 133, 14349. |
| [29] | (c) Wesselbaum S.; Vom Stein T.; Klankermayer J.; Leitner W. Angew. Chem., Int. Ed. 2012, 51, 7499. |
| [29] | (d) Vom Stein T.; Meuresch M.; Limper D.; Schmitz M.; Ho?lscher M.; Coetzee J.; Cole-Hamilton D. J.; Klankermayer J. R.; Leitner W. J. Am. Chem. Soc. 2014, 136, 13217. |
| [30] | Korstanje T. J.; van der Vlugt J. I.; Elsevier C. J.; de Bruin B. Science 2015, 350, 298. |
| [31] | (a) Takebayashi S.; Bergens S. H. Organometallics 2009, 28, 2349. |
| [31] | (b) Kuriyama W.; Ino Y.; Ogata O.; Sayo N.; Saito T. Adv. Synth. Catal. 2010, 352, 92. |
| [31] | (c) Stempfle F.; Quinzler D.; Heckler I.; Mecking S. Macromolecules 2011, 44, 4159. |
| [31] | (d) Furst M. R.; Le Goff R.; Quinzler D.; Mecking S.; Botting C. H.; Cole-Hamilton D. J. Green Chem. 2012, 14, 472. |
| [31] | (e) Fang X.; Zhang C.; Chen J.; Zhu H.; Yuan Y. RSC Adv. 2016, 6, 45512. |
| [31] | (f) Fang X.; Li B.; Zheng J.; Wang X.; Zhu H.; Yuan Y. Dalton Trans. 2019, 48, 2290. |
| [31] | (g) Fang X. L.; Li B.; Jin J.; Duan N. Chin. J. Org. Chem. 2022, 42, 1407 (in Chinese). |
| [31] | (方霄龙, 李斌, 金杰, 段宁, 有机化学, 2022, 42, 1407.) |
| [32] | Van Putten R.; Uslamin E. A.; Garbe M.; Liu C.; Gonzalez‐de‐Castro A.; Lutz M.; Junge K.; Hensen E. J.; Beller M.; Lefort L.; Pidko E. A. Angew. Chem., Int. Ed. 2017, 56, 7531. |
| [33] | (a) Hamilton R. J.; Bergens S. H. J. Am. Chem. Soc. 2006, 128, 13700. |
| [33] | (b) Fang X.; Duan N.; Zhang M.; Zhang C.; Liu R.; Zhu H. Chin. J. Org. Chem. 2019, 39, 1450 (in Chinese). |
| [33] | (方霄龙, 段宁, 章敏, 张春燕, 刘睿, 朱红平, 有机化学, 2019, 39, 1450.) |
| [34] | (a) Langer R.; Iron M. A.; Konstantinovski L.; Diskin‐Posner Y.; Leitus G.; Ben‐David Y.; Milstein D. Chem.-Eur. J. 2012, 18, 7196. |
| [34] | (b) Hoyt J. M.; Shevlin M.; Margulieux G. W.; Krska S. W.; Tudge M. T.; Chirik P. J. Organometallics 2014, 33, 5781. |
| [34] | (c) Lagaditis P. O.; Sues P. E.; Sonnenberg J. F.; Wan K. Y.; Lough A. J.; Morris R. H. J. Am. Chem. Soc. 2014, 136, 1367. |
| [34] | (d) Thai T. T.; Mérel D. S.; Poater A.; Gaillard S.; Renaud J. L. Chem.-Eur. J. 2015, 21, 7066. |
| [34] | (e) Gajewski P.; Renom‐Carrasco M.; Facchini S. V.; Pignataro L.; Lefort L.; de Vries J. G.; Ferraccioli R.; Piarulli U.; Gennari C. Eur. J. Org. Chem. 2015, 2015, 5526. |
| [34] | (f) Rosas-Hernández A.; Alsabeh P. G.; Barsch E.; Junge H.; Ludwig R.; Beller M. Chem. Commun. 2016, 52, 8393. |
| [34] | (g) Hodgkinson R.; Del Grosso A.; Clarkson G.; Wills M. Dalton Trans. 2016, 45, 3992. |
| [35] | Gajewski P.; Gonzalez‐de‐Castro A.; Renom‐Carrasco M.; Piarulli U.; Gennari C.; de Vries J. G.; Lefort L.; Pignataro L. ChemCatChem 2016, 8, 3431. |
| [36] | Azouzi K.; Pedussaut L.; Pointis R.; Bonfiglio A.; Kumari Riddhi R.; Duhayon C.; Bastin S.; Sortais J. B. Organometallics 2023, 42, 1832. |
/
| 〈 |
|
〉 |