

聚集诱导发光活性氟硼吡啶肼醛腙染料的合成、晶体结构及光学性质
收稿日期: 2024-03-22
修回日期: 2024-05-11
网络出版日期: 2024-06-13
基金资助
国家自然科学基金(22271002); 安徽省自然科学基金(2008085QB67); 安徽省自然科学基金(2308085J14); 安徽省高等学校科学研究项目(2023AH050149); 安徽省高等学校科学研究项目(KJ2021A0126); 安徽省教育厅高校优秀科研创新团队(2022AH010073)
Aggregation-Induced Emission (AIE) Active Fluoroboronated Pyridylhydrazinyl Aldehyde Hydrozone Dyes: Synthesis, Crystal Structure and Optical Properties
Received date: 2024-03-22
Revised date: 2024-05-11
Online published: 2024-06-13
Supported by
National Natural Science Foundation of China(22271002); Anhui Provincial Natural Science Foundation(2008085QB67); Anhui Provincial Natural Science Foundation(2308085J14); Scientific Research Project of Anhui Provincial Colleges and Universities(2023AH050149); Scientific Research Project of Anhui Provincial Colleges and Universities(KJ2021A0126); Excellent Scientific Research and Innovation Team in Natural Sciences of Anhui Provincial Department of Education(2022AH010073)
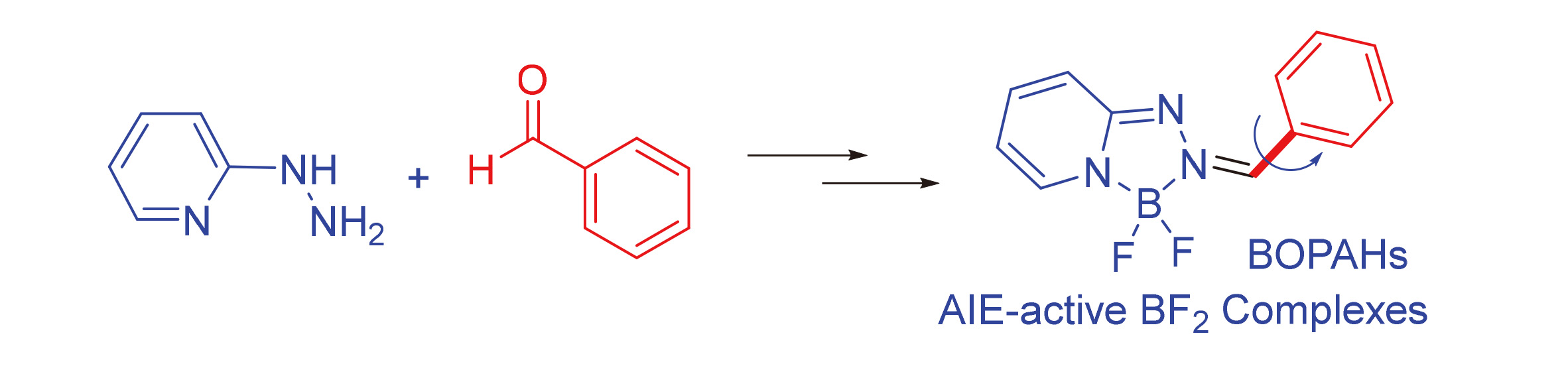
通过简单易得的氯代2-肼基吡啶和芳香醛衍生物缩合与氟硼配位, 一锅两步反应合成了系列新型的氟硼配位吡啶肼基醛腙染料, 将其命名为BOPAHs. 这些染料分子通过核磁共振、高分辨质谱和晶体结构进行了表征. 其主吸收在400~600 nm范围内, 发射波长在500~700 nm范围内. 随着溶剂极性的增加, 它们的吸收/发射峰逐渐红移, 同时斯托克斯位移增大, 表明这些分子具有明显的分子内电荷转移特性. 同时, 通过密度泛函理论计算进一步证实了这一特性. 此外, 这些BOPAHs在溶液中的荧光较弱, 但它们都表现出明显的聚集诱导发射特性, 可能是由于分子在聚集态下构象固定导致弱的分子间相互作用所致.
宫清宝 , 吕翔 , 于长江 , 李婉婉 , 赵全胜 , 焦莉娟 , 郝二红 . 聚集诱导发光活性氟硼吡啶肼醛腙染料的合成、晶体结构及光学性质[J]. 有机化学, 2024 , 44(8) : 2545 -2553 . DOI: 10.6023/cjoc202403032
A new family of fluoroboronated pyridylhydrazinyl aldehyde hydrozone fluorophores named BOPAHs were developed via a simple one-pot two-step reaction from chloro-2-hydrazinylpyridine and aromatic aldehyde derivatives. They were well characterized by NMR, HRMS, and X-ray crystal structures. They exhibit main absorption from 400 nm to 600 nm and emission bands from 500 nm to 700 nm. The absorption/emission bands redshift with increased polarity of solvents indicate a distinct intramolecular charge transfer characteristic, further confirmed by density functional theory (DFT) calculations. These BOPAHs display weak fluorescence in solutions, but they exhibit obvious aggregation-induced emission properties, possibly resulting from weak intermolecular interactions by fixing the molecular conformations in aggregate states.

| [1] | Chen, H.; Han, P.; Qin, A.; Tang, B. Z. Acta Chim. Sinic. 2023, 81, 1420 (in Chinese). |
| [1] | (徐赫, 韩鹏博, 秦安军, 唐本忠, 化学学报, 2023, 81, 1420.) |
| [2] | Mei, J.; Leung, N. L. C.; Kwok, R. T. K.; Lam, J. W. Y.; Tang, B. Z. Chem. Rev. 2015, 115, 11718. |
| [3] | Zhu, F.-Y.; Mei, L.-J.; Tian, R.; Li, C.; Xiang, S.-L.; Zhu, M.-Q.; Wang, Y.-L.; Tang, B. Z. Chem. Soc. Rev. 2024, 53, 3350. |
| [4] | Chen, M.; Dong, R.; Song, J.; Qi, J.; Zhang, J.; Zhao, Z.; Zhang, W.; Li, Y.; Tang, B. Z. Adv. Healthcare Mate.. 2024, 13, 2303967. |
| [5] | Yang, L.-L.; Wang, H.; Zhang, J.; Wu, B.; Li, Q.; Chen, J.-Y.; Tang, A.-L.; Lam, J. W. Y.; Zhao, Z.; Yang, S.; Tang, B. Z. Nat. Commun. 2024, 15, 999. |
| [6] | Chen, Y.-J.; Pu, M.-Q.; Wu, L.-T.; Sun, X.-L.; Wan, W.-M. Chin. J. Chem. 2023, 41, 1705. |
| [7] | Luo, J.; Xie, Z.; Lam, J. W. Y.; Cheng, L.; Chen, H.; Qiu, C.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Z. Chem. Commun. 2001, 18, 1740. |
| [8] | Zhao, Y.; Chen, P.; Li, G.; Niu, Z.; Wang, E. Chin. J. Org. Che.. 2023, 43, 2156 (in Chinese). |
| [8] | (赵洋, 陈盼盼, 李高楠, 钮智刚, 王恩举, 有机化学, 2023, 43, 2156.) |
| [9] | Gui, Y.; Chen, K.; Sun, Y.; Tan, Y.; Luo, W.; Zhu, D.; Xiong, Y.; Yan, D.; Wang, D.; Tang, B. Z. Chin. J. Chem. 2023, 41, 1249. |
| [10] | Long, R.; Tang, C.; Xu, J.; Li, T.; Tong, C.; Guo, Y.; Shi, S.; Wang, D. Chem. Commun. 2019, 55, 10912. |
| [11] | Kim, D.; Lee, U.; Bouffard, J. Kim, Y. Adv. Opt. Mater. 2020, 8, 1902161. |
| [12] | Han, G.; Kim, D.; Park, Y.; Bouffard, J.; Kim, Y. Angew. Che.. 2015, 127, 3984. |
| [13] | Marsh, A. V.; Cheetham, N. J.; Little, M.; Dyson, M.; White, A. J.; Beavis, P.; Warriner, C. N.; Swain, A. C.; Stavrinou, P. N.; Heeney, M. Angew. Che.. 2018, 130, 10800. |
| [14] | Zeng, C.; Hu, P.; Wang, B.; Fang, W.; Zhao, K. Chin. J. Org. Che.. 2023, 43, 3287 (in Chinese). |
| [14] | (曾崇洋, 胡平, 汪必琴, 方文彦, 赵可清, 有机化学, 2023, 43, 3287.) |
| [15] | Huang, W.; Zhao, X.; Zhang, S.; Ying, L.; Miao, X.; Deng, W. Dyes Pig.. 2024, 225, 112063. |
| [16] | Bismillah, A. N.; Aprahamian, I. Chem. Soc. Re.. 2021, 50, 5631. |
| [17] | Mao, Z.; Kim, J. H.; Lee, J.; Xiong, H.; Zhang, F.; Kim, J. S. Coord. Chem. Re.. 2023, 476, 214908. |
| [18] | Chen, Z.; Ni, Z.; Chen, X.-Y.; Xu, Y.; Yu, C.; Wang, S.; Wang, X.-Y.; Lu, H. Aggregat. 2024, 5, e498. |
| [19] | Ji, C.; Yang, J.; Hu, S.; Mack, J.; Zhang, Y.; Lu, H.; Gai, L. Dyes Pig.. 2023, 220, 111707. |
| [20] | Qian, H.; Cousins, M. E.; Horak, E. H.; Wakefield, A.; Liptak, M. D.; Aprahamian, I. Nat. Chem. 2017, 9, 83. |
| [21] | Murali, A. C.; Nayak, P.; Panda, R.; Das, R.; Venkatasubbaiah, K. ACS Appl. Opt. Mate.. 2023, 1, 1033. |
| [22] | Tanaka, K.; Gon, M.; Ito, S.; Ochi, J.; Chujo, Y. Coord. Chem. Re.. 2022, 472, 214779. |
| [23] | Nakamura, M.; Kanetani, I.; Gon, M.; Tanaka, K. Angew. Chem., Int. Ed. 2024, e202404178. |
| [24] | Duan, W.; Liu, Q.; Huo, Y.; Cui, J.; Gong, S.; Liu, Z. Org. Biomol. Chem. 2018, 16, 4977. |
| [25] | Yordanov, D.; Smolka, R.; Nakashima, K.; Hirashima, S. I.; Matsushima, Y.; Vala, M.; Krajcovic, J.; Weiter, M.; Miura, T.; Georgiev, A. J. Org. Che.. 2023, 88, 17206. |
| [26] | Wang, X.; Wu, Y.; Liu, Q.; Li, Z.; Yan, H.; Ji, C.; Duan, J.; Liu, Z. Chem. Commu.. 2015, 51, 784. |
| [27] | Wang, X.; Liu, Q.; Yan, H.; Liu, Z.; Yao, M.; Zhang, Q.; Gong, S.; He, W. Chem. Commun. 2015, 51, 7497. |
| [28] | Liu, Q.; Wang, X.; Yan, H.; Wu, Y.; Li, Z.; Gong, S.; Liu, P.; Liu, Z. J. Mater. Chem. C 2015, 3, 2953. |
| [29] | Wang, H.; Zhang, C.; Jiang, Z.; Xu, L.; Liu, Z. Dalton. Trans. 2023, 52, 1393. |
| [30] | Ni, J.-S.; Zhao, Z.; Liu, H.; Liu, J.; Jiang, M.; Chen, Y.; Kwok, R. T. K.; Lam, J. W. Y.; Peng, Q.; Tang, B. Z. Mater. Chem. Fron.. 2018, 2, 1498 |
| [31] | Yu, C.; Hao, E.; Fang, X.; Wu, Q.; Wang, L.; Li, J.; Xu, L.; Jiao, L.; Wong, W.-Y. J. Mater. Chem. C 2019, 7, 3269. |
| [32] | Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339. |
| [33] | Sheldrick, G. M. Acta Crystallogr. 2015, A71, 3. |
| [34] | Sheldrick, G. M. Acta Crystallogr. 2008, A64, 112. |
| [35] | Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, N. J.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, ?.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision D.01, Gaussian,Inc., Wallingford, C., 2013. |
/
| 〈 |
|
〉 |