

trans-4'a-Methyl-3',4'a,9',10',10'a-hexahydro-spiro[cyclobutane-1,1'(2H)-phenanthrene]的全合成
收稿日期: 2024-04-18
修回日期: 2024-05-26
网络出版日期: 2024-07-02
基金资助
国家重点研发计划(2023YFA1506404); 甘肃省科技计划(23ZDFA003); 甘肃省科技计划(23JRRA1144); 甘肃省科技计划(23JRRA1028); 甘肃省科技计划(23CXGA0043); 兰州市科技计划(2023-1-17); 兰州市科技计划(2023-QN-18); 中央高校基本科研业务专项(lzujbky-2022-ct03); 中央高校基本科研业务专项(lzujbky-2022-sp09); 中央高校基本科研业务专项(lzujbky-2023-ct02); 中央高校基本科研业务专项(lzujbky-2023-pd08); 陇药协同创新中心和甘肃省药物研发计划(2022GSMPA0010)
Total Synthesis of trans-4'a-Methyl-3',4'a,9',10',10'a-hexahydro-spiro[cyclobutane-1,1'(2H)-phenanthrene]
Received date: 2024-04-18
Revised date: 2024-05-26
Online published: 2024-07-02
Supported by
National Key R&D Program of China(2023YFA1506404); Science and Technology Program of Gansu Province(23ZDFA003); Science and Technology Program of Gansu Province(23JRRA1144); Science and Technology Program of Gansu Province(23JRRA1028); Science and Technology Program of Gansu Province(23CXGA0043); Lanzhou Science and Technology Planning Project(2023-1-17); Lanzhou Science and Technology Planning Project(2023-QN-18); Fundamental Research Funds for the Central Universities(lzujbky-2022-ct03); Fundamental Research Funds for the Central Universities(lzujbky-2022-sp09); Fundamental Research Funds for the Central Universities(lzujbky-2023-ct02); Fundamental Research Funds for the Central Universities(lzujbky-2023-pd08); Collaborative Innovation Center for Northwestern Chinese Medicine of Lanzhou University and the Drug Research Project of Gansu Province(2022GSMPA0010)
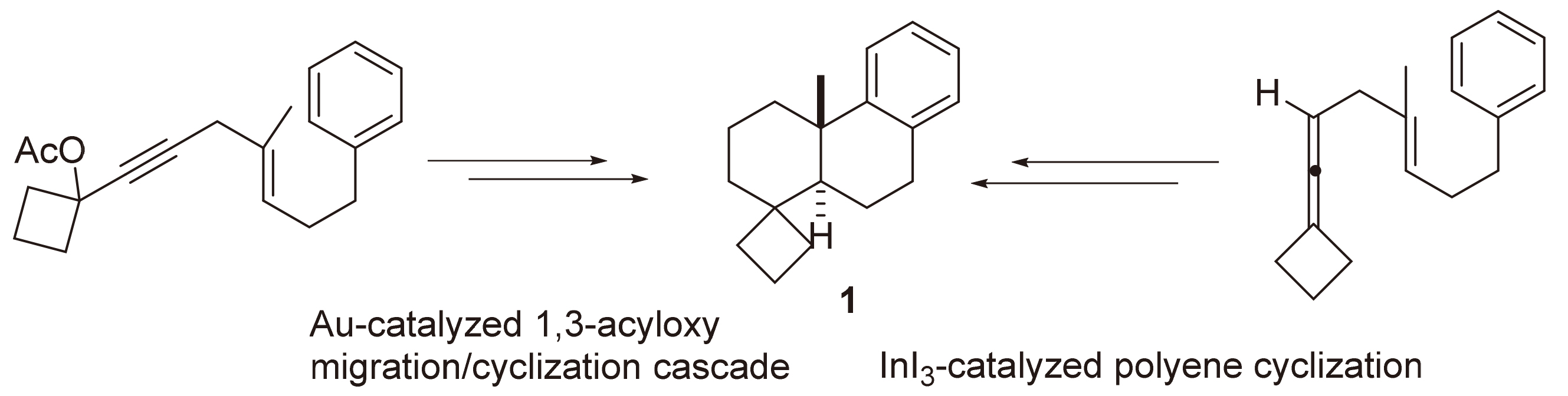
以苯丙醛为起始原料, 通过两种策略的使用完成trans-4'a-methyl-3',4'a,9',10',10'a-hexahydro-spiro[cyclobutane- 1,1'(2H)-phenanthrene]的首次全合成. 具体的关键步骤分别为金催化的1,3-酰基迁移/串联环化和InI3催化的多烯环化.
戴伟昊 , 霍晨雨 , 郑天禄 , 朱道勇 , 王少华 . trans-4'a-Methyl-3',4'a,9',10',10'a-hexahydro-spiro[cyclobutane-1,1'(2H)-phenanthrene]的全合成[J]. 有机化学, 2024 , 44(11) : 3386 -3391 . DOI: 10.6023/cjoc202404026
The efficient total synthesis of trans-4'a-methyl-3',4'a,9',10',10'a-hexahydro-spiro[cyclobutane-1,1'(2H)- phenanthrene] has been accomplished starting from commercially available 3-phenylpropanal by two routes which take Au-catalyzed 1,3-acyloxy migration/cyclization cascade and InI3-catalyzed polyene-type cyclization as the key synthesis steps, respecti- vely.

| [1] | Otvos R. A.; Still K. B. M.; Somsen G. W.; Smit A. B.; Kool J. SLAS Discovery 2019, 24, 362. |
| [2] | Sun D.; Ding H. Chin. J. Org. Chem. 2021, 41, 4827 (in Chinese). |
| [2] | (孙冬宇, 丁寒锋, 有机化学, 2021, 41, 4827.) |
| [3] | Wang P.; Yang C.; Li X.; Jiang Y.; Yan X.; Zhou Z. Chin. J. Org. Chem. 2018, 38, 2199 (in Chinese). |
| [3] | (王平平, 杨成帅, 李晓东, 蒋雨果, 严兴, 周志华, 有机化学, 2018, 38, 2199.) |
| [4] | Song W.; Sun Y.; Xu L.; Sun Y.; Li T.; Peng P.; Lou H. Biorg. Med. Chem. 2021, 29, 115854. |
| [5] | Liu X.; Yu M.; Liang J. Chin. J. Org. Chem. 2023, 43, 1325 (in Chinese). |
| [5] | (刘兴周, 于明加, 梁建华, 有机化学, 2023, 43, 1325.) |
| [6] | Zhao H.; Feng H.; Liu J.; Tang F.; Du Y.; Ji N.; Xie L.; Zhao X.; Wang Z.; Chen Q. Biomaterials 2020, 230, 119598. |
| [7] | Wang N.; Zheng Z.; Jia X.; Zhao M.; Wang Y.; Zhou C.; Wang Z.; Xiao Z.; Liu H.; Ke Y. Chin. J. Org. Chem. 2023, 43, 646 (in Chinese). |
| [7] | (王妮, 郑姿君, 贾小苹, 赵梦圆, 王亚蕾, 周臣, 王志佳, 肖泽霖, 刘宏民, 可钰, 有机化学, 2023, 43, 646.) |
| [8] | Huang L.; Fu Q.; Dai J.-M.; Yan B.-C.; Wang D.; Puno P.-T.; Yue J. Cell Biol. Toxicol. 2021, 37, 695. |
| [9] | Atallah A.; Lecarpentier E.; Goffinet F.; Doret-Dion M.; Gaucherand P.; Tsatsaris V. Drugs 2017, 77, 1819. |
| [10] | Wang J.; Xu C.; Wong Y. K.; Li Y.; Liao F.; Jiang T.; Tu Y. Engineering 2019, 5, 32. |
| [11] | Kanda Y.; Nakamura H.; Umemiya S.; Puthukanoori R. K.; Murthy Appala V. R.; Gaddamanugu G. K.; Paraselli B. R.; Baran P. S. J. Am. Chem. Soc. 2020, 142, 10526. |
| [12] | Ancheeva E.; Daletos G.; Proksch P. Mar. Drugs 2018, 16, 319. |
| [13] | Li D.; Han T.; Liao J.; Hu X.; Xu S.; Tian K.; Gu X.; Cheng K.; Li Z.; Hua H.; Xu J. Int. J. Mol. Sci. 2016, 17, 1395. |
| [14] | Namuli A.; Abdullah N.; Sieo C.; Zuhainis S.; Oskoueian E. J. Med. Plants Res. 2011, 5, 3982. |
| [15] | Gübitz G. M.; Mittelbach M.; Trabi M. Bioresour. Technol. 1999, 67, 73. |
| [16] | Trost B. M.; Scudder P. H. J. Am. Chem. Soc. 1977, 99, 7601. |
| [17] | Gonzalez-Cardenete M. A.; Rivas F.; Basset R.; Stadler M.; Hering S.; Padron J. M.; Zaragoza R. J.; Dea-Ayuela M. A. Antibiotics 2021, 10, 184. |
| [18] | Roa-Linares V. C.; Brand Y. M.; Agudelo-Gomez L. S.; Tangarife-Casta?o V.; Betancur-Galvis L. A.; Gallego-Gomez J. C.; González M. A. Eur. J. Med. Chem. 2016, 108, 79. |
| [19] | Areche C.; Rodriguez J. A.; Razmilic I.; Yanez T.; Theoduloz C.; Schmeda-Hirschmann G. J. Pharm. Pharmacol. 2007, 59, 289. |
| [20] | Bajpai V. K.; Sonwal S.; Hwang S. K.; Shukla S.; Khan I.; Dey D. K.; Chen L.; Simal-Gandara J.; Xiao J.; Huh Y. S.; Han Y. K. Pharmacol. Res. 2021, 163, 105313. |
| [21] | Scariot D. B.; Volpato H.; Fernandes N. S.; Soares E. F. P.; Ueda-Nakamura T.; Dias-Filho B. P.; Din Z. U.; Rodrigues-Filho E.; Rubira A. F.; Borges O.; Sousa M. D. C.; Nakamura C. V. Front. Cell. Infect. Microbiol. 2019, 9, 208. |
| [22] | Wenkert E.; de Paiva Campello J.; McChesney J. D.; Watts D. J. Phytochemistry 1974, 13, 2545. |
| [23] | González M. A. Eur. J. Med. Chem. 2014, 87, 834. |
| [24] | Zhang L.-C.; Wu X.-D.; He J.; Li Y.; Zhang R.-P.; Zhao Q.-S. Phytochem. Lett. 2013, 6, 364. |
| [25] | Cavalcanti A. B. S.; Maia M. S.; Figueiredo P. T. R.; Menezes R. P. B.; Monteiro A. F. M.; Meireles R. A. R.; Rodrigues G. C. S.; Rodrigues de Almeida Silva A. R.; Lins J. S.; Cordeiro L. V.; Junior V. S. R.; Castelo Branco A. P. O. T.; Agra M. d. F.; Sessions Z. L.; Muratov E. N.; Scotti L.; Silva M. S.; Costa V. C. O.; Tavares J. F.; Scotti M. T. Nat. Prod. Res. 2023, 37, 903. |
| [26] | Hirata A.; Kim S. Y.; Kobayakawa N.; Tanaka N.; Kashiwada Y. Fitoterapia 2015, 102, 49. |
| [27] | Matsumoto T.; Usui S.; Kawashima H.; Mitsuki M. Bull. Chem. Soc. Jpn. 1981, 54, 581. |
| [28] | Peters R. J. Nat. Prod. Rep. 2010, 27, 1521. |
| [29] | Wardana A. P.; Aminah N. S.; Rosyda M.; Abdjan M. I.; Kristanti A. N.; Tun K. N. W.; Choudhary M. I.; Takaya Y. Heliyon 2021, 7, e07777. |
| [30] | Li T.; Wu G.; Feng S.; Wang Z.; Xie X.; She X. Org. Biomol. Chem. 2018, 16, 8491. |
| [31] | Barrett A.; Ma T.-K.; Mies T. Synthesis 2019, 51, 67. |
| [32] | Yao L.; Gui J. Chin. J. Org. Chem. 2022, 42, 2703 (in Chinese). |
| [32] | (姚良才, 桂敬汉, 有机化学, 2022, 42, 2703.) |
| [33] | Ungarean C. N.; Southgate E. H.; Sarlah D. Org. Biomol. Chem. 2016, 14, 5454. |
| [34] | Zheng T.-L.; Liu S.-Z.; Huo C.-Y.; Li J.; Wang B.-W.; Jin D.-P.; Cheng F.; Chen X.-M.; Zhang X.-M.; Xu X.-T.; Wang S.-H. CCS Chem. 2020, 3, 2795. |
| [35] | Huo C.-Y.; Zheng T.-L.; Dai W.-H.; Zhang Z.-H.; Wang J.-D.; Zhu D.-Y.; Wang S.-H.; Zhang X.-M.; Xu X.-T. Chem. Sci. 2022, 13, 13893. |
| [36] | Pu X.; Ready J. M. J. Am. Chem. Soc. 2008, 130, 10874. |
/
| 〈 |
|
〉 |