

以N,N-二甲基乙醇胺为单碳合成子构建3,5-二芳基吡啶的新方法
Construction of 3,5-Diarylpyridine Derivatives Using N,N-Dimethyl-ethanolamine as a Single-Carbon Synthon
Received date: 2024-05-07
Revised date: 2024-05-24
Online published: 2024-07-02
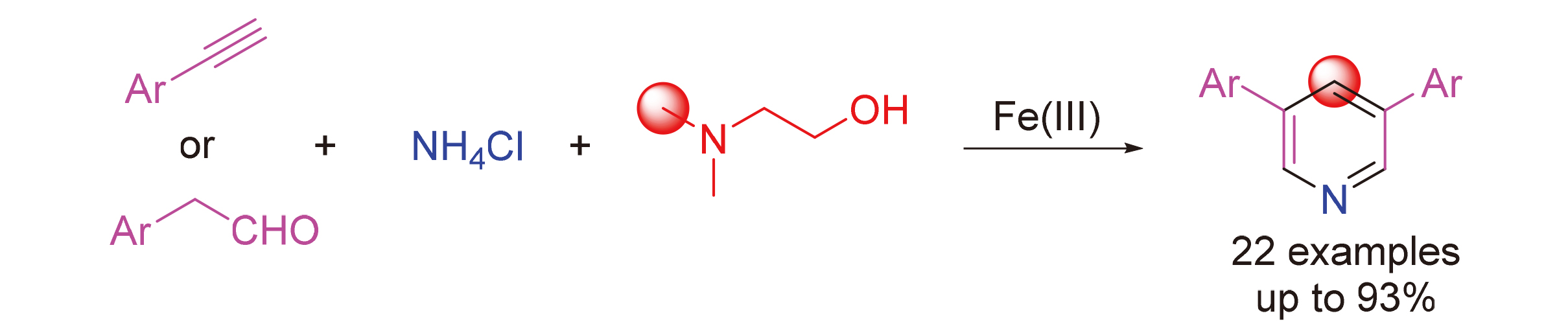
开发了一种以苯乙炔或苯乙醛, 氯化铵和N,N-二甲基乙醇胺为原料, 在140 ℃条件下经三氟甲磺酸铁催化发生[2+2+1+1]环化反应, 其中N,N-二甲基乙醇胺既作为溶剂又作为单碳合成子参与构建3,5二芳基吡啶类化合物.
关键词: 3,5-二芳基吡啶; N,N-二甲基乙醇胺; 单碳合成子; 铁催化; 环合反应
张鑫宇 , 陈静 , 马永敏 . 以N,N-二甲基乙醇胺为单碳合成子构建3,5-二芳基吡啶的新方法[J]. 有机化学, 2024 , 44(11) : 3409 -3416 . DOI: 10.6023/cjoc202405008
3,5-Diarylpyridines were effectively synthesized via [2+2+1+1] cyclization reaction catalyzed by Fe(OTf)3 at 140 ℃, using phenylacetylenes or phenylacetaldehydes, ammonium chloride and N,N-dimethylethanolamine as starting materials. N,N-Dimethylethanolamine was employed as both solvent and a single carbon synthon.

| [1] | (a) Olbe L.; Carlsson E.; Lindberg P. Nat. Rev. Drug Discovery 2003, 2, 132. |
| [1] | (b) Roughley S. D.; Jordan A. M. J. Med. Chem. 2011, 54, 3451. |
| [1] | (c) Altaf A. A.; Shahzad A.; Gul Z.; Rasool N.; Badshah A.; Lal B.; Khan E. J. Drug Des. Med. Chem. 2015, 1, 1. |
| [1] | (d) Hudson G. A.; Hooper A. R.; DiCaprio A. J.; Sarlah D.; Mitchell D. A. Org. Lett. 2021, 23, 253. |
| [1] | (e) Kajita Y.; Ikeda S.; Yoshikawa M.; Fukuda H.; Watanabe E.; Yano J.; Lane W.; Miyamoto M.; Ishii T.; Nishi T.; Koike T. J. Med. Chem. 2022, 65, 3343. |
| [2] | (a) Kumar A.; Rhodes R.; Spychala J.; Wilson W.; Boykin D.; Tidwell R.; Dykstra C.; Hall J.; Jones S.; Schinazi R. Eur. J. Med. Chem. 1995, 30, 99. |
| [2] | (b) Lucas S.; Negri M.; Heim R.; Zimmer C.; Hartmann R. W. J. Med. Chem. 2011, 54, 2307. |
| [2] | (c) Hibi S.; Ueno K.; Nagato S.; Kawano K.; Ito K.; Norimine Y.; Takenaka O.; Hanada T.; Yonaga M. J. Med. Chem. 2012, 55, 10584. |
| [2] | (d) Mohedas A. H.; Wang Y.; Sanvitale C. E.; Canning P.; Choi S.; Xing X.; Bullock A. N.; Cuny G. D.; Yu P. B. J. Med. Chem. 2014, 57, 7900. |
| [2] | (e) Reimann S.; Parpart S.; Ehlers P.; Sharif M.; Spannenberg A.; Langer P. Org. Biomol. Chem. 2015, 13, 6832. |
| [3] | (a) van der Ark A. M.; Verweij A. M. A.; Sinnema A. J. Forensic Sci. 1978, 23, 693. |
| [3] | (b) Tagat J. R.; McCombie S. W.; Barton B. E.; Jackson J. V.; Shortall J. Bioorg. Med. Chem. Lett. 1995, 5, 2143. |
| [3] | (c) Aida W.; Ohtsuki T.; Li X.; Ishibashi M. Tetrahedron 2009, 65, 369. |
| [3] | (d) Carroll F. I.; Ma W.; Deng L.; Navarro H. A.; Damaj M. I.; Martin B. R. J. Nat. Prod. 2010, 73, 306. |
| [3] | (e) Zhang E.; Wang M.; Xu S.; Wang S.; Zhao D.; Bai P.; Cui D.; Hua Y.; Wang Y.; Qin S.; Liu H. Chin. J. Org. Chem. 2017, 37, 959 (in Chinese). |
| [3] | (张恩, 王铭铭, 徐帅民, 王上, 赵娣, 白鹏燕, 崔得运, 化永刚, 王亚娜, 秦上尚, 刘宏民, 有机化学, 2017, 37, 959.) |
| [4] | (a) Fang Z.; Zheng S.; Chan K. F.; Yuan W.; Guo Q.; Wu W.; Lui H. K.; Lu Y.; Leung Y. C.; Chan T. H.; Wong K. Y.; Sun N. Eur. J. Med. Chem. 2019, 161, 141. |
| [4] | (b) Zhao P.; Liu Y.; Zhang Y.; Wang L.; Ma Y. Org. Lett. 2024, 26, 2511. |
| [4] | (c) Zhang R.; Ding Y.; Ma R.; Xiao X.; Chen R.; Wang L.; Ma Y. Org. Chem. Front. 2023, 10, 780. |
| [5] | (a) O'Hagan D. Nat. Prod. Rep. 2001, 17, 435. |
| [5] | (b) De Rycke N.; Couty F.; David O. R. P. Chem.-Eur. J. 2011, 17, 12852. |
| [5] | (c) Zhang R.; Ma R.; Chen R.; Wang L.; Ma Y. J. Org. Chem. 2024, 89, 1846. |
| [5] | (d) Fang J.; Fang J.; Rao Y.; Qiu H.; Pan Z.; Ma Y. Org. Biomol. Chem. 2024, 22, 2043. |
| [5] | (e) Liu Q.; Shi L.; Liu N. P. Chin. J. Org. Chem. 2019, 39, 2882 (in Chinese). |
| [5] | (刘铨瑶, 石磊, 刘宁, 有机化学, 2019, 39, 2882.) |
| [6] | (a) Chelucci G. Chem. Soc. Rev. 2006, 35, 1230. |
| [6] | (b) Gibson V. C.; Redshaw C.; Solan G. A. Chem. Rev. 2007, 107, 1745. |
| [6] | (c) Michaelos T. K.; Shopov D. Y.; Sinha S. B.; Sharninghausen L. S.; Fisher K. J.; Lant H. M. C.; Crabtree R. H.; Brudvig G. W. Acc. Chem. Res. 2017, 50, 952. |
| [6] | (d) Zhang R.; Ma R.; Fu Q.; Chen J.; Ma Y. Chin. J. Org. Chem. 2022, 42, 854 (in Chinese). |
| [6] | (张瑞芹, 马仁超, 傅琴姣, 陈静, 马永敏, 有机化学, 2022, 42, 854.) |
| [7] | (a) Lewis D. E. Angew. Chem., Int. Ed. 2017, 56, 9660. |
| [7] | (b) Yan R.; Zhou X.; Li M.; Li X.; Kang X.; Liu X.; Huo X.; Huang G. RSC Adv. 2014, 4, 50369. |
| [7] | (c) Li Z.; Huang X.; Chen F.; Zhang C.; Wang X.; Jiao N. Org. Lett. 2015, 17, 584. |
| [7] | (d) Al Mehedi M. S.; George D. E.; Tepe J. J. J. Org. Chem. 2022, 87, 16820. |
| [8] | Ranjani G.; Nagarajan R. Org. Lett. 2017, 19, 3974. |
| [9] | Chuang T. H.; Chen Y. C.; Pola S. J. Org. Chem. 2010, 75, 6625. |
| [10] | (a) Komatsu M.; Ohgishi H.; Takamatsu S.; Ohshiro Y.; Agawa T. Angew. Chem., Int. Ed. 2003, 21, 213. |
| [10] | (b) Vijn R. J.; Arts H. J.; Green R.; Castelijns A. Synthesis 1994, 1994, 573. |
| [10] | (c) Balasubrahmanyam S. N.; Jeyashri B.; Namboothiri I. N. N. Tetrahedron 1994, 50, 8127. |
| [11] | Palacios F.; Alonso C.; Rubiales G.; Ezpeleta J. M. Eur. J. Org. Chem. 2001, 2001, 2115. |
| [12] | (a) Sathish M.; Chetna J.; Hari Krishna N.; Shankaraiah N.; Alarifi A.; Kamal A. J. Org. Chem. 2016, 81, 2159. |
| [12] | (b) Li J.; Sun L.; Zhao Y.; Shi C. Chin. J. Org. Chem. 2023, 43, 4168 (in Chinese). |
| [12] | (李进京, 孙立娇, 赵岩, 史成阳, 有机化学, 2023, 43, 4168.) |
| [13] | (a) Wang Q.; Wan C.; Gu Y.; Zhang J.; Gao L.; Wang Z. Green Chem. 2011, 13, 578. |
| [13] | (b) Xiang J. C.; Wang M.; Cheng Y.; Wu A. X. Org. Lett. 2016, 18, 24. |
| [14] | Su M. D.; Liu H. P.; Cao Z. Z.; Liu Y.; Li H.; Nie Z. W.; Yang T. L.; Luo W. P.; Liu Q.; Guo C. C. J. Org. Chem. 2021, 86, 13446. |
| [15] | (a) Mehmood H.; Iqbal M. A.; Ashiq M. N.; Hua R. Molecules 2021, 26, 6599. |
| [15] | (b) Li H.; Yan S.; Xu Y.; Ma C.; Zhang X.; Fan X. Org. Chem. Front. 2024, 11, 1917. |
| [15] | (c) Jia R.; Li B.; Zhang X.; Fan X. Org. Lett. 2020, 22, 6810. |
| [15] | (d) Gao H.; Zhou L.; Wan J. P.; Liu Y. J. Org. Chem. 2023, 88, 7188. |
| [15] | (e) Zhu B.; He J.; Zou K.; Li A.; Zhang C.; Zhao J.; Cao H. J. Org. Chem. 2023, 88, 11450. |
| [16] | (a) Yan M.; Hider R. C.; Ma Y. Org. Chem. Front. 2019, 6, 1168. |
| [16] | (b) Yan M.; Ma R.; Chen R.; Wang L.; Wang Z.; Ma Y. Chem. Commun. 2020, 56, 10946. |
| [16] | (c) Qin Z.; Zhang R.; Ying S.; Ma Y. Org. Chem. Front. 2022, 9, 5624. |
| [16] | (d) Qin Z.; Ma R.; Ying S.; Li F.; Ma Y. Adv. Synth. Catal. 2022, 364, 3263. |
| [16] | (e) Geng M.; Kuang J.; Fang W.; Xiao X.; Ma Y. Org. Chem. Front. 2024, 11, 1198. |
| [17] | (a) Barrios-Bermudez N.; Gonzalez-Avendano M.; Lado-Tourino I.; Cerpa-Naranjo A.; Rojas-Cervantes M. L. Nanomaterials 2020, 10, 749. |
| [17] | (b) Ma B.; Wang S.; Liu F.; Zhang S.; Duan J.; Li Z.; Kong Y.; Sang Y.; Liu H.; Bu W.; Li L. J. Am. Chem. Soc. 2019, 141, 849. |
| [17] | (c) Ma R.; Zhang R.; Xia H.; Wang L.; Ma Y. Eur. J. Org. Chem. 2024, 27, e202400089. |
| [17] | (d) Yamaguchi R.; Kurosu S.; Suzuki M.; Kawase Y. Chem. Eng. J. 2018, 334, 1537. |
| [18] | De Giorgi M.; Voisin-Chiret A. S.; Sopková-de Oliveira Santos J.; Corbo F.; Franchini C.; Rault S. Tetrahedron 2011, 67, 6145. |
| [19] | Avitia B.; MacIntosh E.; Muhia S.; Kelson E. Tetrahedron Lett. 2011, 52, 1631. |
| [20] | Hachey A. C.; Fenton A. D.; Heidary D. K.; Glazer E. C. J. Med. Chem. 2023, 66, 398. |
/
| 〈 |
|
〉 |