

Brønsted碱促进的β-(2-羟基芳基)乙烯磺酰氟与β,γ-不饱和酮酯(炔酮)串联环化反应合成多取代4H-色烯
收稿日期: 2024-05-22
修回日期: 2024-06-08
网络出版日期: 2024-07-10
基金资助
国家自然科学基金(21662029); 石河子大学国际合作(GJHZ202204)
Synthesis of Multisubstituted 4H-Chromenes via Brønsted Base- Mediated Tandem Cyclization Reactions of β-(2-Hydroxyaryl) Ethenesulfonyl Fluorides and β,γ-Unsaturated Ketoesters (Ynones)
Received date: 2024-05-22
Revised date: 2024-06-08
Online published: 2024-07-10
Supported by
National Natural Science Foundation of China(21662029); International Cooperation Project of Shihezi University(GJHZ202204)
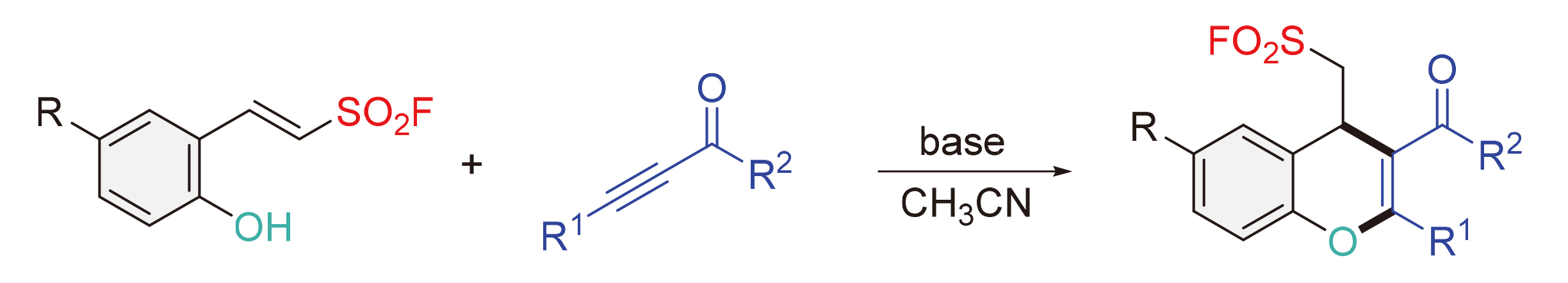
在20 mol% Cs2CO3催化下, β-(2-羟基芳基)乙烯磺酰氟与炔酮酯在室温下发生分子间/分子内Michael加成反应, 以56%~70%的产率获得11种含磺酰氟基团的多取代4H-色烯衍生物. 此外, 在50 mol% Cs2CO3促进下, β-(2-羟基芳基)乙烯磺酰氟与炔酮在50 ℃下发生类似的串联反应, 以25%~80%的产率得到12种多取代的4H-色烯衍生物.
关键词: β-(2-羟基芳基)乙烯磺酰氟; β,γ-不饱和酮酯(炔酮); 4H-色烯
唐德林 , 罗锦昀 , 杜广芬 , 蔡志华 , 何林 . Brønsted碱促进的β-(2-羟基芳基)乙烯磺酰氟与β,γ-不饱和酮酯(炔酮)串联环化反应合成多取代4H-色烯[J]. 有机化学, 2024 , 44(11) : 3365 -3374 . DOI: 10.6023/cjoc202404037
Under the catalysis of 20 mol% Cs2CO3, β-(2-hydroxyaryl) ethenesulfonyl fluorides reacted with ketoesters throu- gh a tandem intermolecular/intramolecular Michael addition process at room temperature, affording 11 multisubstituted 4H- chromenes bearing a useful sulfonyl fluoride groups in 56%~70% yields. In addition, under the mediation of 50 mol% Cs2CO3, β-(2-hydroxyaryl) ethenesulfonyl fluorides couple with ynones through a similar tandem process at 50 ℃, giving 12 multisubstituted 4H-chromenes in 25%~80% yields.

| [1] | (a) Kok T.; Wapenaar H.; Wang K.; Neochoritis C. G.; Zarganes-Tzitzikas T.; Proietti G.; Eleftheriadis N.; Kurpiewska K.; Kalinowska-T?u?cik J.; Cool R. H.; Poelarends G. J.; D?mling A.; Dekker F. J. Bioorg. Med. Chem. 2018, 26, 999. |
| [1] | (b) Mamaghani M.; Nia R. H.; Tavakoli F.; Jahanshahi P. Curr. Org. Chem. 2018, 22, 1704. |
| [1] | (c) Martin E. F.; Mbaveng A. T.; de Moraes M. H.; Kuete V.; Biavatti M. W.; Steindel M.; Efferth T.; Sandjo L. P. Arch. Pharm. 2018, 351, 1800100. |
| [1] | (d) Osipov D. V.; Osyanin V. А. Chem. Heterocycl. Compd. 2021, 57, 505. |
| [2] | Chen C.-C.; Wu M.-Y.; Chen H.-Y.; Wu M.-J. J. Org. Chem. 2017, 82, 6071. |
| [3] | (a) Cossy J.; Menciu C.; Rakotoarisoa H.; Kahn P. H.; Desmurs J.-R. Bioorg. Med. Chem. Lett. 1999, 9, 3439. |
| [3] | (b) Chansakaow S.; Ishikawa T.; Seki H.; Sekine K.; Okada M.; Chaichantipyuth C. J. Nat. Prod. 2000, 63, 173. |
| [4] | Kemnitzer W.; Drewe J.; Jiang S. C.; Zhang H.; Zhao J. H.; Crogan-Grundy C.; Xu L. F.; Lamothe S.; Gourdeau H.; Denis R.; Tseng B.; Kasibhatla S.; Cai S. X. J. Med. Chem. 2007, 50, 2858. |
| [5] | Lee J. W.; Bae S.; Jo W. H. J. Mater. Chem. A 2014, 2, 14146. |
| [6] | (a) Wen Z.; Yang K.-C.; Deng J.-F.; Chen L. Adv. Synth. Catal. 2023, 365, 1290. |
| [6] | (b) Andrade J. S.; Junior P. A. S.; Pereira F. J.; Murta S. M. F.; Correa R. S.; Taylor J. G. Chem. Pap. 2022, 76, 5827. |
| [6] | (c) Katiyar M. K.; Dhakad G. K.; Shivani; Arora S.; Bhagat S.; Arora T.; Kumar R. J. Mol. Struct. 2022, 1263, 133012. |
| [6] | (d) Palapetta S. C.; Kaviyarasan R.; Harichandran G. ChemistrySelect 2023, 8, e202301340. |
| [7] | Nagashima S.; Takahashi T.; Nasrin N.; Kamiguchi S.; Chihara T. Chem. Lett. 2016, 45, 1321. |
| [8] | (a) Li Z.-Y.; Zhang J.-P.; Ying Y.-Y.; Yan D.; Jiao L. J.; Hao E. H. Org. Lett. 2022, 24, 7888. |
| [8] | (b) Gurubrahamam R.; Gao B.-F.; Chen Y. M.; Chan Y.-T.; Tsai M.-K.; Chen K. Org. Lett. 2016, 18, 3098. |
| [8] | (c) Xu Y.-Z.; Tian J.-W.; Sha F.; Li Q. L.; Wu X.-Y. J. Org. Chem. 2021, 86, 6765. |
| [8] | (d) Satbhaiya S.; Khonde N. S.; Rathod J.; Gonnade R.; Kumar P. Eur. J. Org. Chem. 2019, 2019, 3127. |
| [9] | (a) Liu X.; Wang D. Q.; Sun J.; Yan C.-G. J. Mol. Struct. 2024, 1304, 137684. |
| [9] | (b) Suárez-Rodríguez T.; Suárez-Sobrino á. L.; Ballesteros A. Chem.-Eur. J. 2021, 27, 13079. |
| [10] | Wen Z.; Yang K.-C.; Zheng S.-L.; Zhang Y.-S.; Wang S.-J.; Ni H.-L.; Chen L. Org. Biomol. Chem. 2023, 21, 9076. |
| [11] | Chang Z. Q.; Yao J.; Dou X. W. Adv. Synth. Catal. 2020, 362, 3589. |
| [12] | Reddy R. J.; Kumar J. J.; Krishna G. R. Adv. Synth. Catal. 2022, 364, 4080. |
| [13] | (a) Barata-Vallejo S.; Yerien D. E.; Postigo A. Catal. Sci. Technol. 2023, 13, 2597. |
| [13] | (b) Carneiro S. N.; Khasnavis S. R.; Lee J.; Butler T. W.; Majmudar J. D.; am Ende C. W.; Ball N. D. Org. Biomol. Chem. 2023, 21, 1356. |
| [13] | (c) Gambini L.; Udompholkul P.; Salem A. F.; Baggio C.; Pellecchia M. ChemMedChem 2020, 15, 2176. |
| [13] | (d) Meng Y.-P.; Wang S.-M.; Fang W.-Y.; Xie Z.-Z.; Leng J.; Alsulami H.; Qin H.-L. Synthesis 2020, 52, 673. |
| [13] | (e) Zhong T.; Chen Z. D.; Yi J. T.; Lu G.; Weng J. Chin. Chem. Lett. 2021, 32, 2736. |
| [13] | (f) Xu R.; Xu T.; Yang M.; Cao T.; Liao S. Nat. Commun. 2019, 10, 3752. |
| [13] | (g) He F.-S.; Li Y.; Wu J. Org. Chem. Front. 2022, 9, 5299. |
| [14] | (a) Zhang X.; Fang W.-Y.; Qin H.-L. Org. Lett. 2022, 24, 4046. |
| [14] | (b) Zhang F.; An Y.; Liu J. C.; Du G. F.; Cai Z. H.; He L. New J. Chem. 2022, 46, 12367. |
| [14] | (c) Wu Q. S.; Xue Q.; Li J.; Zheng Q. H.; Zhao X. L.; Li W. N.; Sun S. M.; Sha W. X.; Yang Y.; Yang Y.; Li J. P. CCS Chem. 2023, 5, 2251. |
| [15] | (a) Chen X.; Zha G.-F.; Bare G. A. L.; Leng J.; Wang S.-M.; Qin H.-L. Adv. Synth. Catal. 2017, 359, 3254. |
| [15] | (b) Chen X.; Zha G.-F.; Fang W.-Y.; Rakesh K. P.; Qin H.-L. Chem. Commun. 2018, 54, 9011. |
| [15] | (c) Qin H.-L.; Zheng Q. H.; Bare G. A. L.; Wu P.; Sharpless K. B. Angew. Chem., Int. Ed. 2016, 55, 14155. |
| [16] | (a) An Y.; Zhang F.; Du G. F.; Cai Z. H.; He L. Org. Chem. Front. 2021, 8, 6979. |
| [16] | (b) Liu S. J.; Xie P.; Wu L. F.; Zhao J. X.; Cai Z. H.; He L. Org. Chem. Front. 2022, 9, 1550. |
| [16] | (c) Wang W. H.; Wan H. W.; Du G. F.; Dai B.; He L. Org. Lett. 2019, 21, 3496. |
| [16] | (d) Li W.-J.; Pian J.-X.; Gu C.-Z.; Dai B.; He L. Tetrahedron 2020, 76, 131372. |
| [17] | Zhang J.; Liu K.; Jian H.; Li Z.-J.; Yuan J.; He L. Tetrahedron Lett. 2017, 58, 2964. |
| [18] | Pian J. X.; Zhang J.; Li Y. Z.; He L. Chin. Chem. Lett. 2012, 23, 1359. |
| [19] | Li Z. J.; Wang W. H.; Jian H.; Li W. J.; Dai B.; He L. Chin. Chem. Lett. 2019, 30, 386. |
| [20] | (a) Zheng Q. H.; Dong J. J.; Sharpless K. B. J. Org. Chem. 2016, 81, 11360. |
| [20] | (b) Zha G.-F.; Zheng Q. H.; Leng J.; Wu P.; Qin H.-L.; Sharpless K. B. Angew. Chem., Int. Ed. 2017, 56, 4849. |
| [21] | Le Fouler V.; Chen Y.; Gandon V.; Bizet V.; Salomé C.; Fessard T.; Liu F.; Houk K. N.; Blanchard N. J. Am. Chem. Soc. 2019, 141, 15901. |
| [22] | Guo M. J.; Li D.; Zhang Z. G. J. Org. Chem. 2003, 68, 10172. |
/
| 〈 |
|
〉 |