

聚集诱导发光型核酸探针的制备及其核酸传感原理研究
收稿日期: 2024-03-31
修回日期: 2024-05-17
网络出版日期: 2024-07-15
基金资助
国家自然科学基金(21975077); 广东省自然科学基金(2022B1515020084); 广东省分子聚集发光重点实验室开放基金(South China University of Technology, No. 2019B030301003); 华南理工大学发光材料与器件国家重点实验室自主研发项目(Skllmd-2022-01); 广东省基础与应用基础研究基金(2023B1515040003); 云南省科技厅重点项目(202303AC100021)
Preparation of Aggregation-Induced Emission Nucleic Acid Probes and Study of Their Nucleic Acid Sensing Principles
Received date: 2024-03-31
Revised date: 2024-05-17
Online published: 2024-07-15
Supported by
National Natural Science Foundation of China(21975077); Natural Science Foundation of Guangdong Province(2022B1515020084); Open Fund of Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates(South China University of Technology, No. 2019B030301003); Independent Research Project of State Key Lab of Luminescent Materials and Devices (SCUT)(Skllmd-2022-01); Guangdong Basic and Applied Basic Research Foundation(2023B1515040003); Key Project of Yunnan Provincial Department of Science and Technology(202303AC100021)
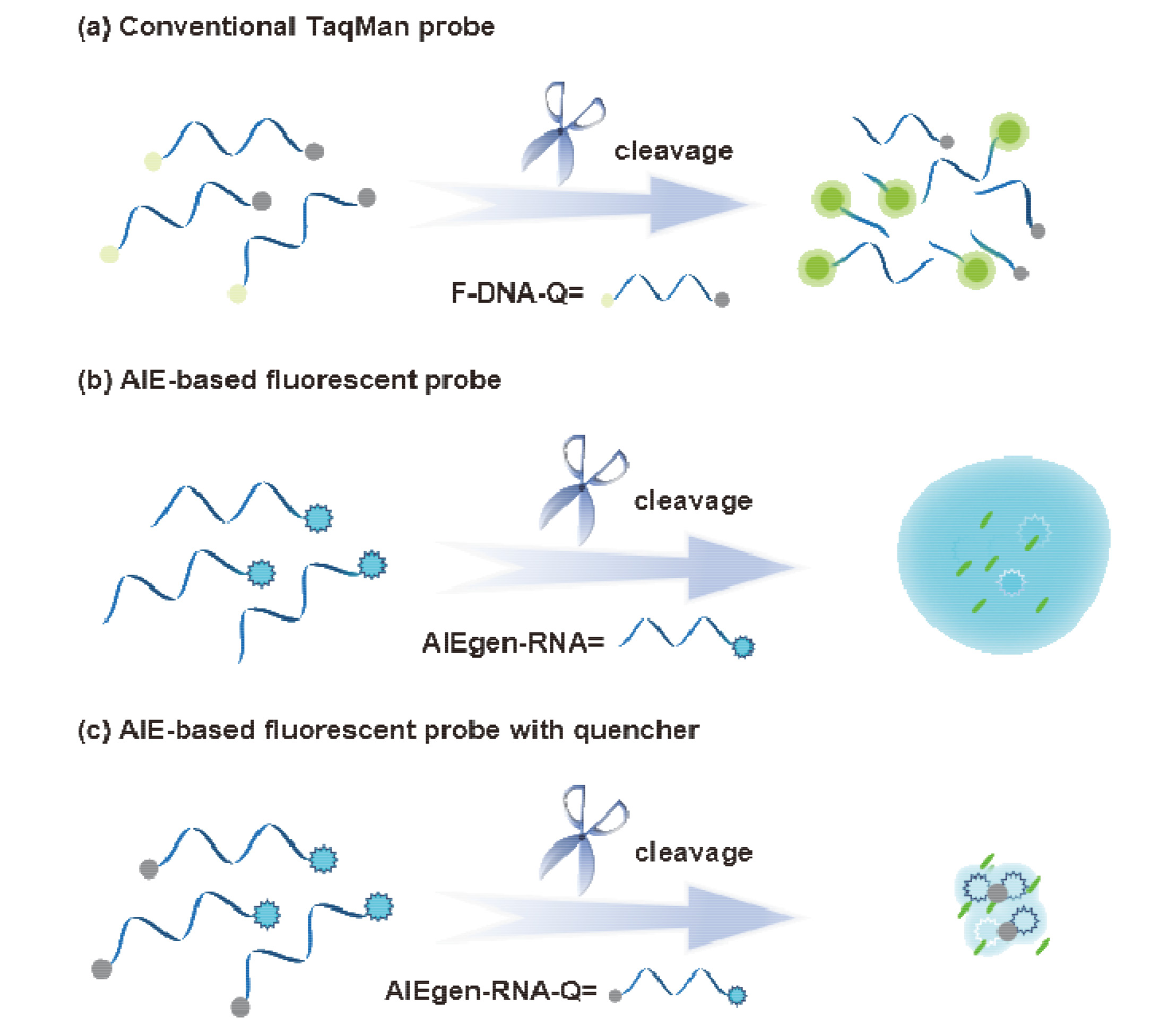
核酸检测是目前生物诊疗及医学分析领域最为精准的方法之一. 其中, 广泛应用的聚合酶链式反应(PCR)技术利用TaqMan探针的荧光信号输出, 可实现对微量目标序列的高灵敏定量分析. 本工作系统性地完成聚集诱导发光(AIE)基元——四苯乙烯(TPE)的亚磷酰胺修饰, 并将其设计和制备成一系列单标记特定RNA的核酸探针, 为后续高效率利用全自动核酸合成仪制备各类AIE探针奠定基础; 利用RNase A酶特异性切割TPE-RNA1探针中水溶性的RNA部分, 通过透射电镜、荧光光谱、质谱等表征证明酶切作用诱发的探针溶解能力变化且伴随着疏水聚集; 通过优化探针浓度、反应时间、序列长度等多个参数, 实现了AIE效应下荧光增强34.7倍. 采用AIE原理取代传统探针中荧光-淬灭基团的双标记策略, 扩展了核酸探针的设计策略, 并系统表征和探讨AIE型核酸探针的聚集过程, 旨在推动更高效、更灵敏的核酸检测探针, 拓展其在生物化学检测领域的应用.
欧彦 , 蓝琳 , 王正雄 , 王志明 , 唐本忠 . 聚集诱导发光型核酸探针的制备及其核酸传感原理研究[J]. 有机化学, 2024 , 44(8) : 2554 -2562 . DOI: 10.6023/cjoc202403057
Nucleic acid detection is one of the most precise methods in biomedicine and medical diagnostics. Among these techniques, the widely employed polymerase chain reaction (PCR) utilizes the fluorescence signal output of TaqMan probes to achieve highly sensitive quantitative analysis of trace target sequences. This study systematically accomplishes the modification of the aggregation-induced emission (AIE) moiety—tetraphenylethylene (TPE) with phosphoramidite, and designs and synthesizes a series of single-labeled nucleic acid probes specific to particular RNA sequences, laying the foundation for the efficient preparation of various AIE nucleic acid probes using automatic oligonucleotide synthesizer. By specifically cleaving the water-soluble RNA segment within the TPE-RNA1 probe using the RNase A enzyme, various characterization techniques including transmission electron microscopy, fluorescence spectroscopy, mass spectrometry, etc., demonstrate changes in probe solubility induced by enzymatic cleavage, accompanied by hydrophobic aggregation. Through the optimization of parameters such as probe concentration, reaction time, and sequence length, a 34.7-fold enhancement in fluorescence under the AIE effect was achieved. This study replaces the traditional dual-labeled strategy of fluorescence-quencher moieties in probes with the AIE principle, expanding the design strategy of nucleic acid probes. It systematically characterizes and discusses the aggregation process of AIE-type nucleic acid probes, aiming to promote more efficient and sensitive nucleic acid detection probes and expand their applications in the field of biochemical detection.

| [1] | Hua, Y.; Ma, J.; Li, D.; Wang, R. Biosensor. 2022, 12, 183. |
| [2] | Samanta, D.; Ebrahimi, S. B.; Mirkin, C. A. Adv. Mater. 2020, 32, 1901743. |
| [3] | Tian, R.; Wang, Z.; Liu, J.; Jiang, Q.; Ding, B. Aggregat. 2021, 2, 133. |
| [4] | Quan, K.; Yi, C.; Yang, X.; He, X.; Huang, J.; Wang, K. TrA., Trends Anal. Chem. 2020, 124, 115784. |
| [5] | Saccà, B.; Niemeyer, C. M. Chem. Soc. Rev. 2011, 40, 5910. |
| [6] | Zhang, X.; Chen, G.; Wang, Y.; Zhao, Y. Innovatio. 2024, 5, 100538. |
| [7] | Fei, X.; Gu, Y. Prog. Nat. Sci. 2009, 19, 1. |
| [8] | Tyagi, S.; Kramer, F. R. Nat. Biotechnol. 1996, 14, 303. |
| [9] | Gibson, U. E.; Heid, C. A.; Williams, P. M. Genome Res. 1996, 6, 995. |
| [10] | Zhang, H.; Huang, F.; Cai, G.; Li, Y.; Lin, J. J. Dairy Sci. 2018, 101, 9736. |
| [11] | Tran, T.; Kostecki, R.; Catton, M.; Druce, J. J. Clin. Microbiol. 2018, 56, e00360-18. |
| [12] | Tabatabaei, M. S.; Islam, R.; Ahmed, M. Anal. Chim. Act. 2021, 1143, 250. |
| [13] | Oxnard, G. R.; Paweletz, C. P.; Kuang, Y.; Mach, S. L.; O'Connell, A.; Messineo, M. M.; Luke, J. J.; Butaney, M.; Kirschmeier, P.; Jackman, D. M.; J?nne, P. A. Clin. Cancer Res. 2014, 20, 1698. |
| [14] | Venkatesan, N.; Jun Seo, Y.; Hyean Kim, B. Chem. Soc. Rev. 2008, 37, 648. |
| [15] | Hwang, G. T.; Seo, Y. J.; Kim, B. H. J. Am. Chem. Soc. 2004, 126, 6528. |
| [16] | Heinlein, T.; Knemeyer, J.-P.; Piestert, O.; Sauer, M. J. Phys. Chem. B 2003, 107, 7957. |
| [17] | Nazarenko, I.; Lowe, B.; Darfler, M.; Ikonomi, P.; Schuster, D.; Rashtchian, A. Nucleic Acids Res. 2002, 30, e37. |
| [18] | Yang, C.; Abbas, F.; Rhouati, A.; Sun, Y.; Chu, X.; Cui, S.; Sun, B.; Xue, C. Biosensor. 2022, 12, 297. |
| [19] | Nikiforov, T. T. Anal. Biochem. 2014, 461, 67. |
| [20] | Knemeyer, J.-P.; Marmé, N.; Sauer, M. Anal. Chem. 2000, 72, 3717. |
| [21] | Sobek, J.; Schlapbach, R. Molecule. 2020, 25, 5369. |
| [22] | Luo, J.; Xie, Z.; Lam, J. W. Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Z. Chem. Commun. 2001, 18, 1740. |
| [23] | Gui, Y.; Chen, K.; Sun, Y.; Tan, Y.; Luo, W.; Zhu, D.; Xiong, Y.; Yan, D.; Wang, D.; Tang, B. Z. Chin. J. Chem. 2023, 41, 1249. |
| [24] | Lu, H.; Ma, L.; Ma, H. Chin. J. Org. Chem. 2023, 43, 4075 (in Chinese). |
| [24] | (鲁会名, 马拉毛草, 马恒昌, 有机化学, 2023, 43, 4075.) |
| [25] | Xu, H.; Han, P.; Qin, A.; Tang, B. Z. Acta Chim. Sinic. 2023, 81, 1420 (in Chinese). |
| [25] | (徐赫, 韩鹏博, 秦安军, 唐本忠, 化学学报, 2023, 81, 1420.) |
| [26] | Feng, X.; Zhu, L.; Yue, B. Acta Chim. Sinic. 2022, 80, 647 (in Chinese). |
| [26] | (冯锡成, 朱亮亮, 岳兵兵, 化学学报, 2022, 80, 647.) |
| [27] | Zhao, Y.; Chen, P.; Li, G.; Niu, Z.; Wang, E. Chin. J. Org. Chem. 2023, 43, 2156 (in Chinese). |
| [27] | (赵洋, 陈盼盼, 李高楠, 钮智刚, 王恩举, 有机化学, 2023, 43, 2156.) |
| [28] | Zhang, Y.; Nie, F.; Zhou, L.; Wang, X.; Liu, Y.; Huo, Y.; Chen, W.; Zhao, Z. Chin. J. Org. Chem. 2023, 43, 3876 (in Chinese). |
| [28] | (张越华, 聂飞, 周路, 王晓烽, 刘源, 霍延平, 陈文铖, 赵祖金, 有机化学, 2023, 43, 3876.) |
| [29] | Zhu, L.; Zhou, J.; Xu, G.; Li, C.; Ling, P.; Liu, B.; Ju, H.; Lei, J. Chem. Sci. 2018, 9, 2559. |
| [30] | Ma, K.; Zhang, F.; Sayyadi, N.; Chen, W.; Anwer, A. G.; Care, A.; Xu, B.; Tian, W.; Goldys, E. M.; Liu, G. ACS Sens. 2018, 3, 320. |
| [31] | Wang, X.; Dai, J.; Min, X.; Yu, Z.; Cheng, Y.; Huang, K.; Yang, J.; Yi, X.; Lou, X.; Xia, F. Anal. Chem. 2018, 90, 8162. |
| [32] | Chen, J.; Jiang, H.; Zhou, H.; Hu, Z.; Niu, N.; Shahzad, S. A.; Yu, C. Chem. Commun. 2017, 53, 2398. |
| [33] | Lu, D.; He, L.; Wang, Y.; Xiong, M.; Hu, M.; Liang, H.; Huan, S.; Zhang, X.-B.; Tan, W. Talant. 2017, 167, 550. |
| [34] | Min, X.; Zhuang, Y.; Zhang, Z.; Jia, Y.; Hakeem, A.; Zheng, F.; Cheng, Y.; Tang, B. Z.; Lou, X.; Xia, F. ACS Appl. Mater. Interface. 2015, 7, 16813. |
| [35] | Li, Y.; Kwok, R. T. K.; Tang, B. Z.; Liu, B. RSC Adv. 2013, 3, 10135. |
| [36] | Chen, Z.; Wei, Z.; Xiao, F.; Chao, Z.; Lu, J.; Wang, Z.; Tian, L. Adv. Funct. Mater. 2022, 32, 2207845. |
| [37] | Beaucage, S. L.; Caruthers, M. H. Tetrahedron Lett. 1981, 22, 1859. |
| [38] | Krotz, A. H.; Rentel, C.; Gorman, D.; Olsen, P.; Gaus, H. J.; McArdle, J. V.; Scozzari, A. N. Nucleoside., Nucleotides Nucleic Acids 2004, 23, 767. |
| [39] | Takamoto, K.; He, Q.; Morris, S.; Chance, M. R.; Brenowitz, M. Nat. Struct. Mol. Biol. 2002, 9, 928. |
/
| 〈 |
|
〉 |