

双(三氟甲磺酰亚胺)钙催化1-取代氨基-2-芳基乙烯基1H-吲哚-1-羧酸酯的合成
收稿日期: 2024-04-06
修回日期: 2024-05-20
网络出版日期: 2024-07-15
基金资助
国家自然科学基金(21772156)
Calcium Trifluoromethanesulfonimide Catalyzed Synthesis of 1-Substituted-amino-2-arylvinyl 1H-Indole-1-carboxylates
Received date: 2024-04-06
Revised date: 2024-05-20
Online published: 2024-07-15
Supported by
National Natural Science Foundation of China(21772156)
基于双(三氟甲磺酰亚胺)钙[Ca(NTf2)2]催化炔酰胺与1-吲哚甲酸叔丁酯的加成反应, 建立了一种高区域选择性制备1-取代氨基-2-芳基乙烯基1H-吲哚-1-羧酸酯的合成方法. 经过15个底物的验证, 该方法的收率稳定在52%~87%之间, 区域选择性高.
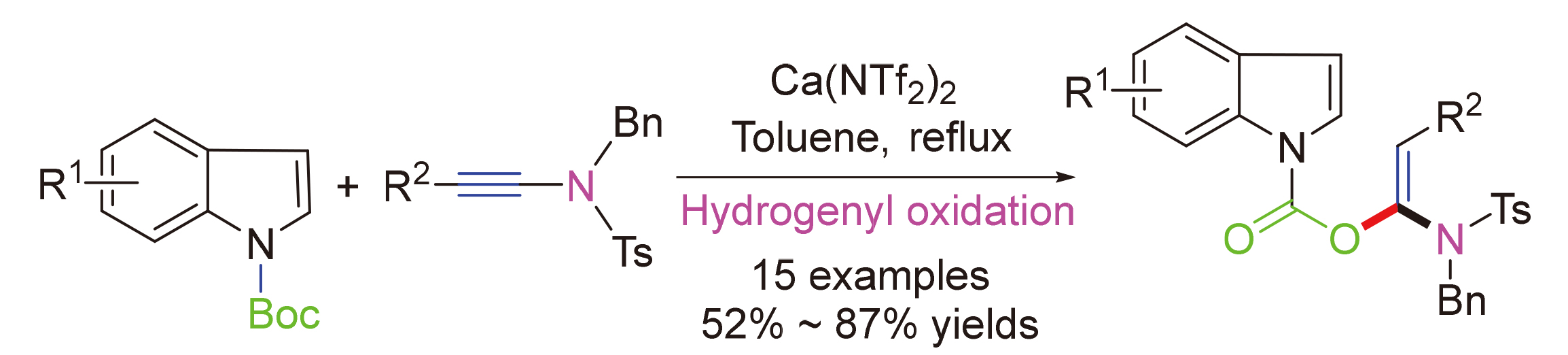
关键词: 双(三氟甲磺酰亚胺)钙; 炔酰胺; 吲哚; 1H-吲哚-1-羧酸酯; 区域选择性
张育莹 , 宋庆燕 , 李月容 , 郑怀基 , 魏邦国 . 双(三氟甲磺酰亚胺)钙催化1-取代氨基-2-芳基乙烯基1H-吲哚-1-羧酸酯的合成[J]. 有机化学, 2024 , 44(11) : 3490 -3496 . DOI: 10.6023/cjoc202404007
An efficient approach to access 1-substituted-amino-2-arylvinyl 1H-indole-1-carboxylates was achieved through calcium trifluoromethanesulfonimide [Ca(NTf2)2] catalyzed addition process of ynamides with tert-butyl 1-indolecarboxylate. After verification with 15 substrates, the yields of this method were stable between 52% and 87%, and regioselectivities were excellent.

| [1] | (a) Huang H.; Zhang T.; Sun J. Angew. Chem., Int. Ed. 2021, 60 2668. |
| [1] | (b) Jin L.; Zhou X.; Zhao Y.; Guo J.; Stephan D. W. Org. Biomol. Chem. 2022, 20, 7781. |
| [1] | (c) Staderini M.; Bolognesi M. L.; Menéndez J. C. Adv. Synth. Catal. 2014, 357, 185. |
| [2] | (a) Apostolopoulos V.; Bojarska J.; Chai T. T.; Elnagdy S.; Kaczmarek K.; Matsoukas J.; New R.; Parang K.; Lopez O. P.; Parhiz H. Molecules 2021, 26, 430. |
| [2] | (b) Fosgerau K.; Hoffmann T. Drug Discovery Today 2015, 20, 122. |
| [2] | (c) Davyt D.; Entz W.; Fernandez R.; Mariezcurrena R.; Mombrú A. W.; Saldana J.; Domínguez L.; Coll J.; Manta E. J. Nat. Prod. 1998, 61, 1560. |
| [2] | (d) Huang L.; Arndt M.; Goo?en K. T.; Heydt H.; Goossen L. J. Chem. Rev. 2015, 115, 2596. |
| [3] | (a) Patel J. P.; Repta A. Int. J. Pharm. 1981, 9, 29. |
| [3] | (b) Ilankumaran P.; Verkade J. G. J. Org. Chem. 1999, 64, 9063. |
| [3] | (c) Hara H.; Hirano M.; Tanaka K. Org. Lett. 2008, 10, 2537. |
| [4] | (a) Superchi S.; Sotomayor N.; Miao G.; Joseph B.; Campbell M. G.; Snieckus V. Tetrahedron Lett. 1996, 37, 6061. |
| [4] | (b) Panella L.; Feringa B. L.; de Vries J. G.; Minnaard A. J. Org. Lett. 2005, 7, 4177. |
| [5] | (a) Liu W.; Lan H.; Huang J. B.; Liu W.; Jiang K. Z.; Xiao X.; Ni S. F.; Liu J.; Bai Y.; Shao X. Org. Lett. 2024, 26, 687. |
| [5] | (b) Afanasenko A. M.; Wu X.; De Santi A.; Elgaher W. A.; Kany A. M.; Shafiei R.; Schulze M. S.; Schulz T. F.; Haupenthal J.; Hirsch A. K. Angew. Chem., Int. Ed. 2024, 136, e202308131. |
| [6] | (a) Chien P. C.; Chen Y. R.; Chen Y. J.; Chang C. F.; Marri G.; Lin W. Adv. Synth. Catal. 2024, 366, 420. |
| [6] | (b) Lin Z.; Li M.; Yoshioka R.; Oyama R.; Kabe R. Angew. Chem., Int. Ed. 2024, 63, e202314500. |
| [7] | Kawade R. K.; Tseng C. C.; Liu R. S. Chem.-Eur. J. 2014, 20, 13927. |
| [8] | Smith D. L.; Goundry W. R.; Lam H. W. Chem. Commun. 2012, 48, 1505. |
| [9] | (a) Xu S.; Liu J.; Hu D.; Bi X. Green Chem. 2015, 17, 184. |
| [9] | (b) Hu L.; Xu S.; Zhao Z.; Yang Y.; Peng Z.; Yang M.; Zhao J. J. Am. Chem. Soc. 2016, 138, 13135. |
| [10] | (a) Habert L.; Retailleau P.; Gillaizeau I. Org. Biomol. Chem. 2018, 16, 7351. |
| [10] | (b) Habert L.; Sallio R.; Durandetti M.; Gosmini C.; Gillaizeau I. Eur. J. Org. Chem. 2019, 2019, 5175. |
| [11] | Han P.; Mao Z. Y.; Li M.; Si C. M.; Wei B. G.; Lin G. Q. J. Org. Chem. 2020, 85, 4740. |
| [12] | Takebe H.; Yoshino N.; Shimada Y.; Williams C. M.; Matsubara S. Org. Lett. 2023, 25, 27. |
| [13] | (a) Sagamanova I. Org. Synth. 2007, 84, 359. |
| [13] | (b) Zhang X.; Li H.; You L.; Tang Y.; Hsung R. P. Adv. Synth. Catal. 2006, 348, 2437. |
| [13] | (c) Zhang X.; Zhang Y.; Huang J.; Hsung R. P.; Kurtz K. C.; Oppenheimer J.; Petersen M. E.; Sagamanova I. K.; Shen L.; Tracey M. R. J. Org. Chem. 2006, 71, 4170. |
| [13] | (d) Zhang Y.; Hsung R. P.; Tracey M. R.; Kurtz K. C.; Vera E. L. Org. Lett. 2004, 6, 1151. |
/
| 〈 |
|
〉 |