

钯/膦配合物催化Morita-Baylis-Hillman碳酸酯与氮杂二烯的[4+4]环化反应
Palladium/Phosphine Complex Catalyzed [4+4] Annulations of Morita-Baylis-Hillman Carbonates and 1-Azadienes
Received date: 2024-05-15
Revised date: 2024-06-21
Online published: 2024-07-25
Supported by
National Natural Science Foundation of China(21971166)
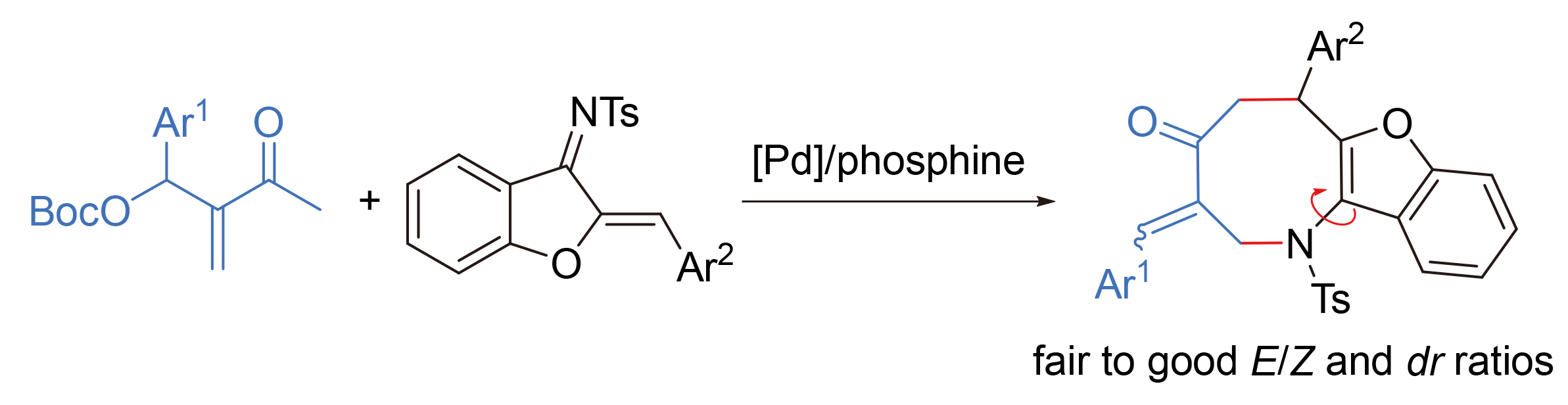
在醋酸钯和膦配体Synphos配合物的催化下, 从醛衍生的Morita-Baylis-Hillman (MBH)加成物碳酸酯可原位生成含π-烯丙基钯的1,4-全碳偶极子, 与1-氮杂二烯发生串联Michael加成/烯丙位取代反应, 构建一系列含有三取代环外双键的八元氮杂环产物, 并取得了中等到良好的E/Z选择性和阻转选择性. 此外, 在手性配体或者手性季铵盐的协同催化下能够实现中等的对映选择性控制.
朱波 , 杨杨 , 刘淇尹 , 杜玮 , 陈应春 . 钯/膦配合物催化Morita-Baylis-Hillman碳酸酯与氮杂二烯的[4+4]环化反应[J]. 有机化学, 2024 , 44(12) : 3761 -3770 . DOI: 10.6023/cjoc202405020
A [4+4] annulation reaction for the construction of medium-sized N-heterocycles is reported. This process involves the generation of all-carbon 1,4-dipoles containing a π-allylpalladium complex from Morita-Baylis-Hillman (MBH) carbonates under the catalysis of Pd/Synphos, which then undergo Michael addition/N-allylic alkylation with 1-azadienes. A spectrum of eight-membered N-heterocycles featuring a trisubstituted exo-cyclic double bond is furnished efficiently with moderate to good E/Z selectivity and moderate atroposelectivity. In addition, moderate enantioselectivity can be realized by using a chiral ligand or with the assistant of a chiral quaternary ammonium salt.

| [1] | (a) Laakso J. A.; Gloer J. B.; Wicklow D. T.; Dowd P. F. J. Org. Chem. 1992, 57, 2066. |
| [1] | (b) Lee S.; Sperry J. Bioorg. Med. Chem. 2022, 54, 116560. |
| [1] | (c) Toma T.; Kita Y.; Fukuyama T. J. Am. Chem. Soc. 2010, 132, 10233. |
| [1] | (d) Qin H.; Xu Z.; Cui Y.; Jia Y. Angew. Chem., Int. Ed. 2011, 50, 4447. |
| [1] | (e) Miloserdov F. M.; Kirillova M. S.; Muratore M. E.; Echavarren A. M. J. Am. Chem. Soc. 2018, 140, 5393. |
| [2] | (a) Reyes R. L.; Iwai T.; Sawamura M. Chem. Rev. 2021, 121, 8926. |
| [2] | (b) Wang Y.-H.; Jin Z.-F.; Zhou L.J.; Lv X. Org. Biomol. Chem. 2024, 22, 252. |
| [2] | (c) Li J.; Dong Z.-Y.; Zhao C.-G. New J. Chem. 2024, 48, 4645. |
| [3] | (a) Illuminati G.; Mandolini L. Acc. Chem. Res. 1981, 14, 95. |
| [3] | (b) Molander G.-A. Acc. Chem. Res. 1998, 31, 603. |
| [4] | (a) Zhang M.-M.; Qu B.-L.; Shi B.; Xiao W.-J.; Lu L.-Q. Chem. Soc. Rev. 2022, 51, 4146. |
| [4] | (b) Wang B.-C.; Wei Y.; Xiong F.-Y.; Qu B.-L.; Xiao W.-J.; Lu L.-Q. Sci. China Chem. 2022, 65, 2437. |
| [4] | (c) Shi L.; Xiong Q.; Wu S.-Y.; Li Y.; Shen P.; Lu J.; Ran G.-Y. Org. Lett. 2023, 25, 2030. |
| [4] | (d) Wang L.; Yang S.; Tang Y.; Li K.; Lu M.-X.; Guo H.-C. J. Org. Chem. 2024, 89, 5019. |
| [5] | (a) Li Q.-Y.; Pan R.; Wang M.-H.; Yao H.-Q.; Lin A.-J. Org. Lett. 2021, 23, 2292. |
| [5] | (b) Scuiller A.; Liu X.-Y.; Cordier M.; Garrec J.; Archambeau A. Synlett 2021, 32, 981. |
| [5] | (c) Song Q.; Liu Y.; Cai L.-L.; Cao X.-Y.; Qian S.; Wang Z.-Y. Chin. Chem. Lett. 2022, 33, 4549. |
| [5] | (d) Meng Y.-G.; Wang Q.; Yao X.-Y.; Wei D. H.; Liu Y.-G.; Li E.-Q.; Duan Z. Org. Lett. 2022, 24, 9205. |
| [5] | (e) Chen G.-H.; Ye Y.; Zhang D.-X.; Li H.-G.; Zhang N.; Liang G.-J.; Zhang D.; Zhou J.; Zhou H. Org. Chem. Front. 2023, 10, 4698. |
| [5] | (f) Xie H.-L.; Chen L.; Han Z.-Y.; Yang Z.-K.; Sun J.-W.; Huang H. Org. Lett. 2023, 25, 5011. |
| [5] | (g) Yuan C.-H.; Wu Y.; Wang D.-Q.; Zhang Z.-H.; Wang C.; Zhou L.-J.; Zhang C.; Song B.-A.; Guo H.-C. Adv. Synth. Catal. 2018, 360, 652. |
| [5] | (h) Niu B.; Wu X.-Y.; Wei Y.; Shi M. Org. Lett. 2019, 21, 4859. |
| [5] | (i) Yang W.-L.; Wang Y.-L.; Li W.; Gu B.-M.; Wang S.-W.; Luo X.-Y.; Tian B.-X.; Deng W.-P. ACS Catal. 2021, 11, 12557. |
| [5] | (j) Xu H.-B.; Ma S.-M. Angew. Chem., Int. Ed. 2023, 62, e202213676. |
| [6] | Gao C.; Wang H.-H.; Liu J.-T.; Li X.-X. ACS Catal. 2021, 11, 2684. |
| [7] | Gao Y.-F.; Wang H.; Chen X.-Q.; Qiao Y.-Y.; Miao Z.-Y. J. Org. Chem. 2023, 88, 11822. |
| [8] | (a) Chen Y.; Zang M.; Wang W.-J.; Liu Y.-Z.; Luo X.-Y.; Deng W.-P. Chin. J. Chem. 2023, 41, 2825. |
| [8] | (b) Chen G.-H.; Ye Y.; Zhang D.-X.; Li H.-J.; Zhang N.; Liang G.-J.; Zhang D.; Zhou J.; Zhou H. Org. Chem. Front. 2023, 10, 4698. |
| [9] | Yang Y.; Zhu B.; Zhu L.; Jiang Y.; Guo C.-L.; Gu J.; Ouyang Q.; Du W.; Chen Y.-C. Chem. Sci. 2021, 12, 11399. |
| [10] | (a) Lin W.; Zhang C.; Xu W.; Cheng Y.-Y.; Li P.-F.; Li W.-J. Adv. Synth. Catal. 2019, 361, 476. |
| [10] | (b) Hu D.; Gao Y.; Song X.; Du W.; Chen Y.-C. Eur. J. Org. Chem. 2020, 514. |
| [10] | (c) Jiang B.; Du W.; Chen Y.-C. Chem. Commun. 2020, 56, 7257. |
| [10] | (d) Yan J.-Z.; Li X.-P.; Chen Y.-Z.; Li Y.; Chen W.-W.; Zhan R.-T.; Huang H.-C. J. Org. Chem. 2020, 85, 12175. |
| [10] | (e) Wang C.-J. Yang Q.-Q.; Wang M.-X.; Shang Y.-H.; Tong X.-H.; Deng Y.-H.; Shao Z.-H. Org. Chem. Front. 2020, 7, 609. |
| [10] | (f) Yang X.-X.; Yan R.-J.; Ran G.-Y.; Chen C.; Yue J.-F.; Yan X.; Ouyang Q.; Du W.; Chen Y.-C. Angew. Chem., Int. Ed. 2021, 60, 26762 |
| [10] | (g) Luo Q.-Q.; Tian Z.; Tang J.; Wang J.; Tian Y.; Peng C.; Zhan G.; Han B. ACS Catal. 2022, 12, 7221. |
| [10] | (h) Yang T.; Jiang Q.; Wang C.-M.; Li S.-L.; He C.-Y.; Chu W.-D.; Liu Q.-Z. Org. Lett. 2023, 25, 13, 2243. |
| [11] | (a) Tabata H.; Wada N.; Takada Y.; Oshitari T.; Takahashi H.; Natsugari H. J. Org. Chem. 2011, 76, 5123. |
| [11] | (b) Wang L.; Li S.; Marcus.; Arne R. P.; Wang A.; Rakesh P.; Kari R.; Dieter E. Angew. Chem., Int. Ed. 2016, 128, 11276. |
| [12] | For more details, see the Supporting Information. |
| [13] | (a) Xie P.-Z.; Huang Y. Org. Biomol. Chem. 2015, 13, 8578. |
| [13] | (b) Liu T.-Y.; Xie M.; Chen Y.-C. Chem. Soc. Rev. 2012, 41, 4101. |
| [14] | (a) Ohmatsu K.; Imagawa N.; Ooi T. Nat. Chem. 2014, 6, 47. |
| [14] | (b) Ohmatsu K.; Kawai S.; Imagawa N.; Ooi T. ACS Catal. 2014, 4, 4304. |
| [15] | Deposition numbers 2355492 (E-3a) and 2355493 (Z-3a) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre. |
/
| 〈 |
|
〉 |