

光催化胺与二硫化碳合成硫脲
收稿日期: 2024-05-07
修回日期: 2024-07-02
网络出版日期: 2024-09-02
基金资助
国家自然科学基金(21901179)
Synthesis of Thioureas from Amines and CS2 via Photoredox Catalysis
Received date: 2024-05-07
Revised date: 2024-07-02
Online published: 2024-09-02
Supported by
National Natural Science Foundation of China(21901179)
李伟 , 王奕森 , 周荣 , 高文超 . 光催化胺与二硫化碳合成硫脲[J]. 有机化学, 2025 , 45(1) : 240 -245 . DOI: 10.6023/cjoc202405007
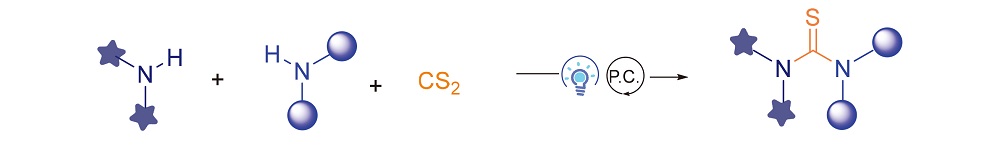
In recent years, photocatalysis has become an important tool to construct new compounds in synthetic and pharmaceutical chemistry. In this work, various symmetric and unsymmetric thioureas were successfully synthesized by photoredox catalysis using CS2 as thiocarbonyl source. For the mechanism, thiyl radicals were generated via single electron transfer process under photoredox catalysis after the nucleophilic addition of amines to CS2, and then dimerized to reactive disulfides, which reacted with the other amines to form thioureas. The method features mild conditions, good functional group tolerance, and simple operation.
Key words: photochemistry; thioureas; carbon disulfide; green synthesis
| [1] | Mahanta, N.; Szantai-Kis, M.; Petersson, E. J.; Mitchell, D. A. ACS Chem. Biol. 2019, 14, 142. |
| [2] | Gurdal, E. E.; Durmaz, I.; Cetin-Atalay, R. J. Enzyme Inhib. Med. Chem. 2014, 29, 205. |
| [3] | Thanh, N. D.; Giang, N. T. K.; Toan, V. N.; Van, T. K. H.; Tri, N. M.; Toanae, D. N. New J. Chem. 2023, 47, 22360. |
| [4] | Klein, J. J.; Hecht, S. Org. Lett. 2012, 14, 330. |
| [5] | Nedeljković, N.; Nikolić, M.; Čanović, P.; Milan, Z.; Zarić, R. Ž.; Bošković, J.; Vesović, M.; Bradić, J.; Anđić, M.; Kočović, A.; Nikolić, M.; Jakovljević, V.; Vujić, Z.; Dobričić, V. Pharmaceutics 2023, 16, 1. |
| [6] | Liu, J.; Liao, P.; Hu, J.; Zhu, H.; Wang, Y.; Li, Y.; Li, Y.; He, B. Molecules 2017, 22, 238. |
| [7] | Kumar, K. N.; Reddy, M. M.; Panchami, H.; Velayutham, R.; Dhaked, D. K.; Swain, S. P. Mol. Catal. 2022, 524, 112324. |
| [8] | Lee, H.; Nam, H.; Lee, S. Y. J. Am. Chem. Soc. 2024, 146, 3065. |
| [9] | Zhou, X.-Y.; Li, X.-Y.; Zhang, Z.; Yu, D.-G. Chin. Chem. Lett. 2021, 32, 4015. |
| [10] | Li, X.-Y.; Liu, Y.; Chen, X.-L.; Lu, X.-Y.; Liang, X.-X.; Zhu, S.-S.; Wei, C.-W.; Qu, L.-B.; Yu, B. Green Chem. 2020, 22, 4445. |
| [11] | Wu, Y.; Lin, Y.-W.; He, W.-M. Chin. Chem. Lett. 2020, 31, 2999. |
| [12] | Ding, C.; Wang, S.; Sheng, Y.; Dai, Q.; Zhao, Y.; Liang, G.; Song, Z. RSC Adv. 2019, 9, 26768. |
| [13] | Zhong, P.; Wu, J.; Liu, J.-B.; Luo, N. Tetrahedron Lett. 2022, 108, 154143. |
| [14] | Phaenok, S.; Nguyen, L. A.; Soorukram, D.; Nguyen, T. T. T.; Retailleau, P.; Nguyen, T. B. Chem.-Eur. J. 2024, 30, e202303703. |
| [15] | (a) Su, Y.; Zou, Y.; Xiao, W. Chin. J. Org. Chem. 2022, 42, 3201 (in Chinese). |
| [15] | (苏艺雯, 邹有全, 肖文精, 有机化学, 2022, 42, 3201.) |
| [15] | (b) Xu, H.; Zhang, J.; Zuo, J.; Wang, F.; Lü, J.; Hun, X.; Yang, D. Chin. J. Org. Chem. 2022, 42, 4037 (in Chinese). |
| [15] | (徐浩, 张杰, 左峻泽, 王丰晓, 吕健, 混旭, 杨道山, 有机化学, 2022, 42, 4037.) |
| [15] | (c) Pu, J.; Jia, X.; Han, L.; Li, Q. Chin. J. Org. Chem. 2023, 43, 2591 (in Chinese). |
| [15] | (普佳霞, 贾小英, 韩丽荣, 李清寒, 有机化学, 2023, 43, 2591.) |
| [16] | Liu, H.; Zhao, L.; Yuan, Y.; Xu, Z.; Chen, K.; Qiu, S.; Tan, H. ACS Catal. 2016, 6, 1732. |
| [17] | Gao, W.-C.; Li, W.; Zhang, J.; Chang, H.-H.; Zhou, R. Green Synth. Catal. 2024, 10.1016/j.gresc.2024.02.008. |
/
| 〈 |
|
〉 |