

碳-碳不饱和键的催化不对称氢-膦(磷)官能化反应研究进展
收稿日期: 2024-05-30
修回日期: 2024-09-01
网络出版日期: 2024-09-10
基金资助
国家自然科学基金(22171029); 四川省自然科学基金(2024NSFSC0281)
Advances in Catalytic Asymmetric Hydrogen-Phosphine/Phosphorus Functionalization of Unsaturated Carbon-Carbon Bonds
Received date: 2024-05-30
Revised date: 2024-09-01
Online published: 2024-09-10
Supported by
National Natural Science Foundation of China(22171029); Natural Science Foundation of Sichuan Province(2024NSFSC0281)
手性含膦(磷)化合物广泛应用于医药、农业、材料以及不对称催化合成等领域, 因此, 发展高效方法用于合成结构多样的手性含膦(磷)化合物引起许多化学工作者的关注. 在已发展的众多合成策略中, 碳-碳不饱和键的催化不对称氢-膦(磷)官能化反应是一类高效且原子经济的方法. 利用该方法可以高效地合成多种类型的手性含膦(磷)化合物, 包括含有碳手性、轴手性、磷手性以及同时含有碳手性和磷手性中心的含膦(磷)化合物. 主要介绍了近十年碳-碳不饱和键的催化不对称氢-膦(磷)官能化反应的研究进展, 从反应机理、合成应用、存在的挑战以及未来的发展等方面进行了阐释与讨论.
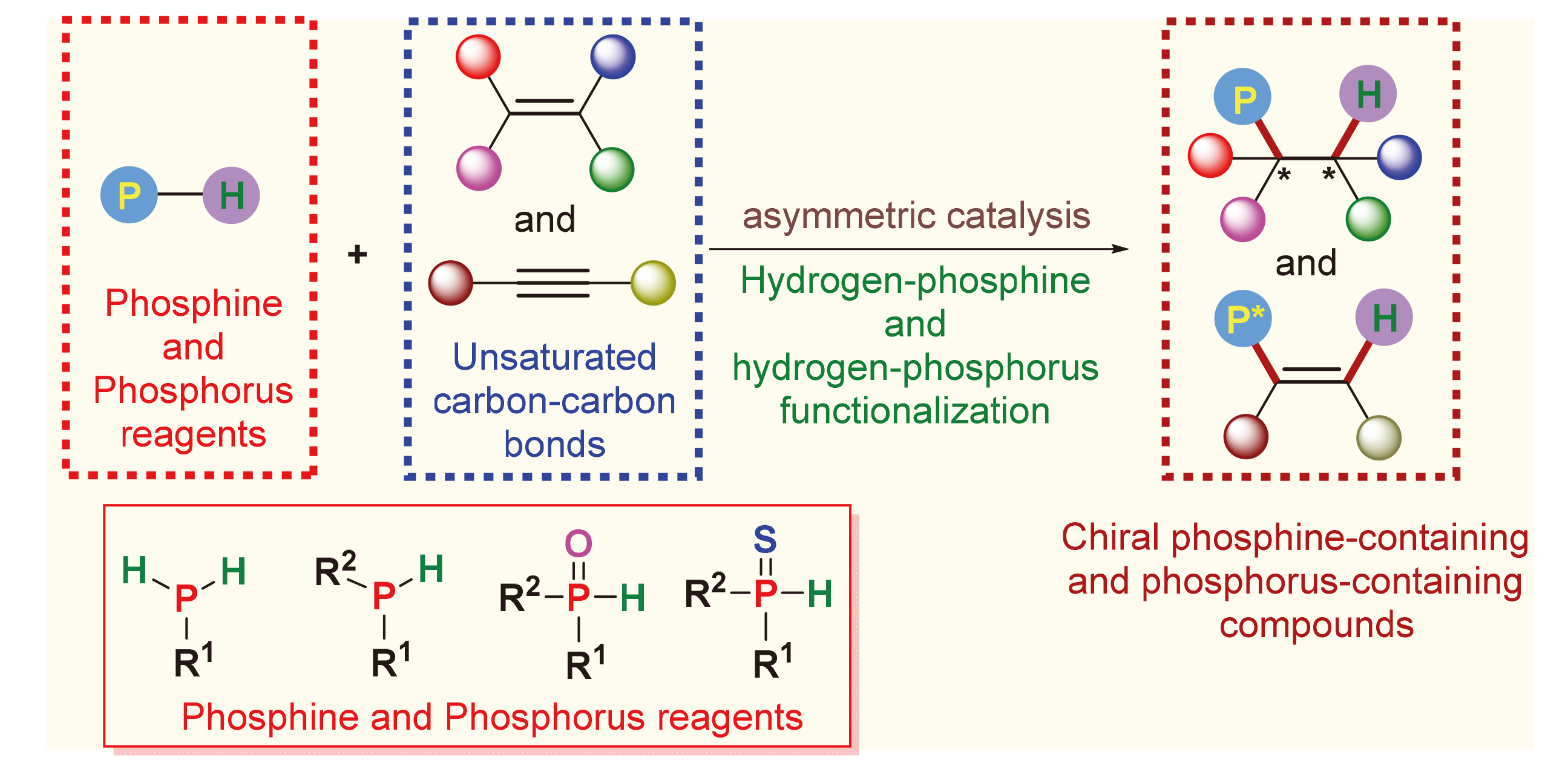
关键词: 不对称催化; 碳-碳不饱和键; 氢-膦(磷)官能化; 手性含膦(磷)化合物; 膦(磷)试剂
孙婷珈 , 孙国银 , 孙威 , 彭雪松 , 廖娟 , 游勇 , 袁伟成 . 碳-碳不饱和键的催化不对称氢-膦(磷)官能化反应研究进展[J]. 有机化学, 2024 , 44(12) : 3647 -3677 . DOI: 10.6023/cjoc202405046
Phosphine/Phosphorus-containing chiral compounds have a wide range of applications in pharmaceuticals industry, agriculture, functionalized materials, and asymmetric catalysis. Accordingly, the development of efficient methods for the synthesis of structurally diverse phosphine/phosphorus-containing chiral compounds has attracted the attention of chemists. Among the multitude of synthetic strategies developed, catalytic asymmetric hydrogen-phosphine/phosphorus functiona- lization of unsaturated carbon-carbon bonds stands out as a particularly efficient and atom economical method. Utilizing this strategy, a wide array of phosphine/phosphorus-containing chiral compounds can be synthesized with remarkable efficiency. The state-of-the-art studies on catalytic asymmetric hydrogen-phosphine/phosphorus functionalization of unsaturated carbon- carbon bonds in past decade are summarized. Mechanistic insights, synthetic applications, as well as challenges and opportunities of this field are elucidated and discussed.

| [1] | (a) Hecker S.-J.; Erion M.-D. J. Med. Chem. 2008, 51, 2328. |
| [1] | (b) Sheng X.-C.; Pyun H.-J.; Chaudhary K.; Wang J.; Doerffler E.; Fleury M.; Kim C.-U. Bioorg. Med. Chem. Lett. 2009, 19, 3453. |
| [1] | (c) Zhang Y.-P.; You Y.; Yin J.-Q.; Wang Z.-H.; Zhao J.-Q.; Li Q.; Yuan W.-C. Eur. J. Org. Chem. 2023, 26, e202300728. |
| [1] | (d) Wiemer A.-J. ACS Pharmacol. Transl. Sci. 2020, 3, 613. |
| [1] | (e) Jia X.; Schols D.; Meier C. J. Med. Chem. 2020, 63, 6003. |
| [1] | (f) Atherton F. R.; Hassall C. H.; Lambert R. W. J. Med. Chem. 1986, 29, 29. |
| [1] | (g) Lavielle G.; Hautefaye P.; Schaeffer C.; Boutin J. A.; Cudennec C. A.; Pierre A. J. Med. Chem. 1991, 34, 1998. |
| [2] | (a) Xu G.-Q.; Senanayake C.-H.; Tang W.-J. Acc. Chem. Res. 2019, 52, 1101. |
| [2] | (b) Li W.-B.; Zhang J.-L. Chem. Soc. Rev. 2016, 45, 1657. |
| [3] | (a) Yao Q.-L.; Wang A.-J.; Pu J.-Z.; Tang Y.-M. Chin. J. Org. Chem. 2014, 34, 292 (in Chinese). |
| [3] | (姚秋丽, 王安俊, 蒲家志, 唐瑜敏, 有机化学, 2014, 34, 292.) |
| [3] | (b) Li Z.; Duan W.-L. Chin. J. Org. Chem. 2016, 36, 1805 (in Chinese). |
| [3] | (李振, 段伟良, 有机化学, 2016, 36, 1805.) |
| [3] | ((c) Zhu R.-Y.; Liao K.; Yu J.-S.; Zhou J. Acta Chim. Sinica 2020, 78, 193 (in Chinese). |
| [3] | (朱仁义, 廖奎, 余金生, 周剑, 化学学报, 2020, 78, 193.) |
| [3] | (d) Li H.; Yin L. Chin. J. Org. Chem. 2022, 42, 3183 (in Chinese). |
| [3] | (李晖, 殷亮, 有机化学, 2022, 42, 3183.) |
| [3] | (e) Luo C.; Yin Y.-L.; Jiang Z.-Y. Chin. J. Org. Chem. 2023, 43, 1963 (in Chinese). |
| [3] | (罗诚, 尹艳丽, 江智勇, 有机化学, 2023, 43, 1963.) |
| [3] | (f) Ding K.; Su B. Eur. J. Org. Chem. 2024, 27, e202301160. |
| [4] | (a) Feng J.-J.; Chen X.-F.; Shi M.; Duan W.-L. J. Am. Chem. Soc. 2010, 132, 5562. |
| [4] | (b) Huang Y.-H.; Pullarkat S. A.; Li Y.-X.; Leung P.-H. Chem. Commun. 2010, 46, 6950. |
| [5] | Wang C.-Y.; Huang K.-S.; Ye J.; Duan W.-L. J. Am. Chem. Soc. 2021, 143, 5685. |
| [6] | Hao X.-Q.; Zhao Y.-W.; Yang J.-J.; Niu J.-L.; Gong J.-F. Song M.-P. Organometallics 2014, 33, 1801. |
| [7] | Li C.; Bian Q.-L.; Xu S.; Duan W.-L. Org. Chem. Front. 2014, 1, 541. |
| [8] | Song Y.-C.; Dai G.-F.; Xiao F.-H.; Duan W.-L. Tetrahedron Lett. 2016, 57, 2990. |
| [9] | Chen Y.-R.; Feng J.-J.; Duan W.-L. Tetrahedron Lett. 2014, 55, 595. |
| [10] | Chew R. J.; Lu Y.-P.; Jia Y.-X.; Li B.-B.; Wong E. H. Y.; Goh R.; Li Y.-X.; Huang Y.-H.; Pullarkat S. A.; Leung P.-H. Chem.- Eur. J. 2014, 20, 14514. |
| [11] | Chew R. J.; Sepp K.; Li B.-B.; Li Y.-X.; Zhu P.-C.; Tan N. S.; Leung P.-H. Adv. Synth. Catal. 2015, 357, 3297. |
| [12] | Chew R. J.; Teo K. Y.; Huang Y.-H.; Li B.-B.; Li Y.-X.; Pullarkat S. A.; Leung P.-H. Chem. Commun. 2014, 50, 8768. |
| [13] | Xu Y.; Yang Z.-H.; Ding B.-Q.; Liu D.-L.; Liu Y.-G.; Sugiya M.; Imamoto T.; Zhang W.-B. Tetrahedron 2015, 71, 6832. |
| [14] | Yue W.-J.; Xiao J.-Z.; Zhang S.; Yin L. Angew. Chem., Int. Ed. 2020, 59, 7057. |
| [15] | Li Y.-B.; Tian H.; Yin L. J. Am. Chem. Soc. 2020, 142, 20098. |
| [16] | Pérez J. M.; Postolache R.; Reis M. C.; Sinnema E. G.; Vargová D.; Vries F.; Otten E.; Ge L.; Harutyunyan S. R. J. Am. Chem. Soc. 2021, 143, 20071. |
| [17] | Ge L.; Harutyunyan S. R. Chem. Sci. 2022, 13, 1307. |
| [18] | Postolache R.; Pérez J. M.; Reis M. C.; Ge L.; Sinnema E. G.; Harutyunyan S. R. Org. Lett. 2023, 25, 1611. |
| [19] | Wang C.-Y.; Yin P.; Dai Y.-H.; Ye J.; Duan W.-L. J. Organomet. Chem. 2023, 983, 122552. |
| [20] | Ji D.-Q.; Qi Z.-S.; Li X.-W. Org. Lett. 2023, 25, 5957. |
| [21] | Yang Q.-J.; Zhou J.; Wang J. Chem. Sci. 2023, 14, 4413. |
| [22] | Sinnema E. G.; Ramspoth T.-F.; Bouma R. H.; Ge L.; Harutyunyan S. R. Angew. Chem., Int. Ed. 2024, 63, e202316785. |
| [23] | Lu J.-Z.; Ye J.-X.; Duan W.-L. Chem. Commun. 2014, 50, 698. |
| [24] | Huang J.; Zhao M.-X.; Duan W.-L. Tetrahedron Lett. 2014, 55, 629. |
| [25] | Huang Y.-H.; Li Y.-X.; Leung P.-H.; Hayashi T. J. Am. Chem. Soc. 2014, 136, 4865. |
| [26] | Sadeer A.; Ong Y. J.; Kojima T.; Foo C. Q.; Li Y.-X.; Pullarkat S.-A.; Leung P.-H. Chem.-Asian J. 2018, 13, 2829. |
| [27] | Lu Z.-W.; Zhang H.-Y.; Yang Z.-P.; Ding N.; Meng L.; Wang J. ACS Catal. 2019, 9, 1457. |
| [28] | Zhang Y.-L.; Jiang Y.-X.; Li M.-L.; Huang Z.-X.; Wang J. Chem. Catal. 2022, 2, 3163. |
| [29] | Lin X.-B.; An K.; Zhuo Q.-D.; Nishiura M.-Y.; Cong X.-F.; Hou Z.-M. Angew. Chem., Int. Ed. 2023, 62, e202308488. |
| [30] | Zhang S.; Jiang N.; Xiao J.-Z.; Lin G.-Q.; Yin L. Angew. Chem., Int. Ed. 2023, 62, e202218798. |
| [31] | Zhang Y.-D.; Zhu S.-F. Acta Chim. Sinica 2023, 81, 777 (in Chinese). |
| [31] | (张艳东, 朱守非, 化学学报, 2023, 81, 777.) |
| [32] | Ji D.-Q.; Jing J.-R.; Wang Y.; Qi Z.-S.; Wang F.; Zhang X.-P.; Wang Y.; Li X.-W. Chem 2022, 8, 3346. |
| [33] | Wu Z.-H.; Cheng A.-Q.; Yuan M.; Zhao Y.-X.; Yang H.-L.; Wei L.-H.; Wang H.-Y.; Wang T.; Zhang Z.-T.; Duan W.-L. Angew. Chem., Int. Ed. 2021, 60, 27241. |
| [34] | Yu X.-H.; Lu L.-Q.; Zhang Z.-H.; Shi D.-Q.; Xiao W.-J. Org. Chem. Front. 2023, 10, 133. |
| [35] | Nie S.-Z.; Davison R.-T.; Dong V.-M. J. Am. Chem. Soc. 2018, 140, 16450. |
| [36] | Long J.; Li Y.-Q.; Zhao W.-N.; Yin G.-Y. Chem. Sci. 2022, 13, 1390. |
| [37] | (a) Li G.-L.; Huo X.-H.; Jiang X.-Y.; Zhang W.-B. Chem. Soc. Rev. 2020, 49, 2060. |
| [37] | (b) Blieck R.; Taillefer M.; Monnier F. Chem. Rev. 2020, 120, 13545. |
| [38] | Yang Z.-P.; Wang J. Angew. Chem., Int. Ed. 2021, 60, 27288. |
| [39] | Zhou J.; Meng L.; Lin S.-J.; Cai B.-H.; Wang J. Angew. Chem., Int. Ed. 2023, 62, e202303727. |
| [40] | Duan S.-Z.; Pan A.-L.; Du Y.; Zhu G.-L.; Tian X.; Zhang H.-B.; Walsh P. J.; Yang X.-D. ACS Catal. 2023, 13, 10887. |
| [41] | Dai Q.; Liu L.; Qian Y.-Y.; Li W.-B.; Zhang J.-L. Angew. Chem., Int. Ed. 2020, 59, 20645. |
| [42] | Xie X.-X.; Li S.-L.; Chen Q.-Y.; Guo H.; Yang J.-F.; Zhang J.-L. Org. Chem. Front. 2022, 9, 1589. |
| [43] | Yang Z.-P.; Gu X.-D.; Han L.-B.; Wang J. Chem. Sci. 2020, 11, 7451. |
| [44] | Liu X.-T.; Han X.-Y.; Wu Y.; Sun Y.-Y.; Gao L.; Huang Z.; Zhang Q.-W. J. Am. Chem. Soc. 2021, 143, 11309. |
| [45] | Wang W.-H.; Wu Y.; Qi P.-J.; Zhang Q.-W. ACS Catal. 2023, 13, 6994. |
| [46] | Zhang Y.-Q.; Han X.-Y.; Wu Y.; Qi P.-J.; Zhang Q.; Zhang Q.-W. Chem. Sci. 2022, 13, 4095. |
| [47] | (a) Ananikov V. P.; Makarov A. V.; Beletskaya I. P. Chem.-Eur. J. 2011, 17, 12623. |
| [47] | (b) Ananikov V. P.; Beletskaya I. P. Chem.-Asian J. 2011, 6, 1423. |
| [47] | (c) Khemchyan L. L.; Ivanova J. V.; Zalesskiy S. S.; Ananikov V. P.; Beletskaya I. P.; Starikova Z. A. Adv. Synth. Catal. 2014, 356, 771. |
| [48] | Cai B.-H.; Cui Y.; Zhou J.; Wang Y.-B.; Yang L.-M.; Tan B.; Wang J. Angew. Chem., Int. Ed. 2023, 62, e202215820. |
| [49] | Li Y.-B.; Li Y.; Yin L. Chin. Chem. Lett. 2024, 35, 109294. |
| [50] | Maiti R.; Yan J.-L.; Yang X.; Mondal B.; Xu J.; Chai H.-F.; Jin Z.-C.; Robin Chi Y.-G. Angew. Chem., Int. Ed. 2021, 60, 26616. |
| [51] | Gu X.; Yuan H.; Jiang J.; Wu Y.; Bai W.-J. Org. Lett. 2018, 20, 7229. |
| [52] | Chen Y.; Yu Z.-Y.; Jiang Z.-Y.; Tan J.-P.; Wu J.-H.; Lan Y.; Ren X.-Y.; Wang T.-L. ACS Catal. 2021, 11, 14168. |
| [53] | Jia Y.-Q.; Zhao J.-Q.; Wang Z.-H.; You Y.; Zhang Y.-P.; Jin X.; Zhou M.-Q.; Ge Z.-Z.; Yuan W.-C. Chem. Commun. 2022, 58, 12062. |
| [54] | Arai R.; Hirashima S.-I.; Nakano T.; Kawada M.; Akutsu H.; Nakashima K.; Miura T. J. Org. Chem. 2020, 85, 3872. |
| [55] | (a) Shi Y.-R.; Chen L.-R.; Gao Q.; Li J.-L.; Guo Y.-F.; Fan B.-M. Org. Lett. 2023, 25, 6495. |
| [55] | (b) Qian J.-Y.; Zhao H.-Y.; Gao Q.; Chen L.-R.; Shi Y.-R.; Li J.-L.; Guo Y.-F.; Fan B.-M. Org. Chem. Front. 2023, 10, 5672. |
| [55] | (c) Chen L.-R.; Wang G.-Y.; Nong X.-F.; Shao W.-D.; Li J.-L.; Guo Y.-F.; Fan B.-M. Chem.-Eur. J. 2024, e202401017. |
| [56] | Kondoh A.; Ishikawa S.; Terada M. Org. Biomol. Chem. 2020, 18, 7814. |
| [57] | Das S.; Hu Q.-P.; Kondoh A.; Terada M. Angew. Chem., Int. Ed. 2021, 60, 1417. |
| [58] | Wang B.-H.; Liu Y.-L.; Jiang C.-Y.; Cao Z.; Cao S.-S.; Zhao X.-W.; Ban X.; Yin Y.-L.; Jiang Z.-Y. Angew. Chem., Int. Ed. 2023, 62, e202216605. |
| [59] | Hirashima S.-I.; Hirota E.; Matsushima Y.; Noda N.; Nishimura Y.; Narushima T.; Nakashima K.; Miura T. Chem.-Asian J. 2022, 17, e202200989. |
/
| 〈 |
|
〉 |