

过渡金属催化远程二烯的不对称迁移烯丙位碳氢键官能团化
收稿日期: 2024-06-29
修回日期: 2024-07-26
网络出版日期: 2024-09-10
基金资助
国家自然科学基金(22071262); 国家自然科学基金(22371292); 上海市科学技术委员会(22ZR1475200); 宁波市自然科学基金(2023J036); 中国科学院战略先导研究计划(XDB0610000)
Transition Metal-Catalyzed Asymmetric Migratory Allylic C—H Functionalization of Remote Dienes
Received date: 2024-06-29
Revised date: 2024-07-26
Online published: 2024-09-10
Supported by
National Natural Science Foundation of China(22071262); National Natural Science Foundation of China(22371292); Science and Technology Commission of Shanghai Municipality(22ZR1475200); Natural Science Foundation of Ningbo(2023J036); Strategic Priority Research Program of the Chinese Academy of Sciences(XDB0610000)
张经明 , 何智涛 . 过渡金属催化远程二烯的不对称迁移烯丙位碳氢键官能团化[J]. 有机化学, 2025 , 45(2) : 592 -601 . DOI: 10.6023/cjoc202406047
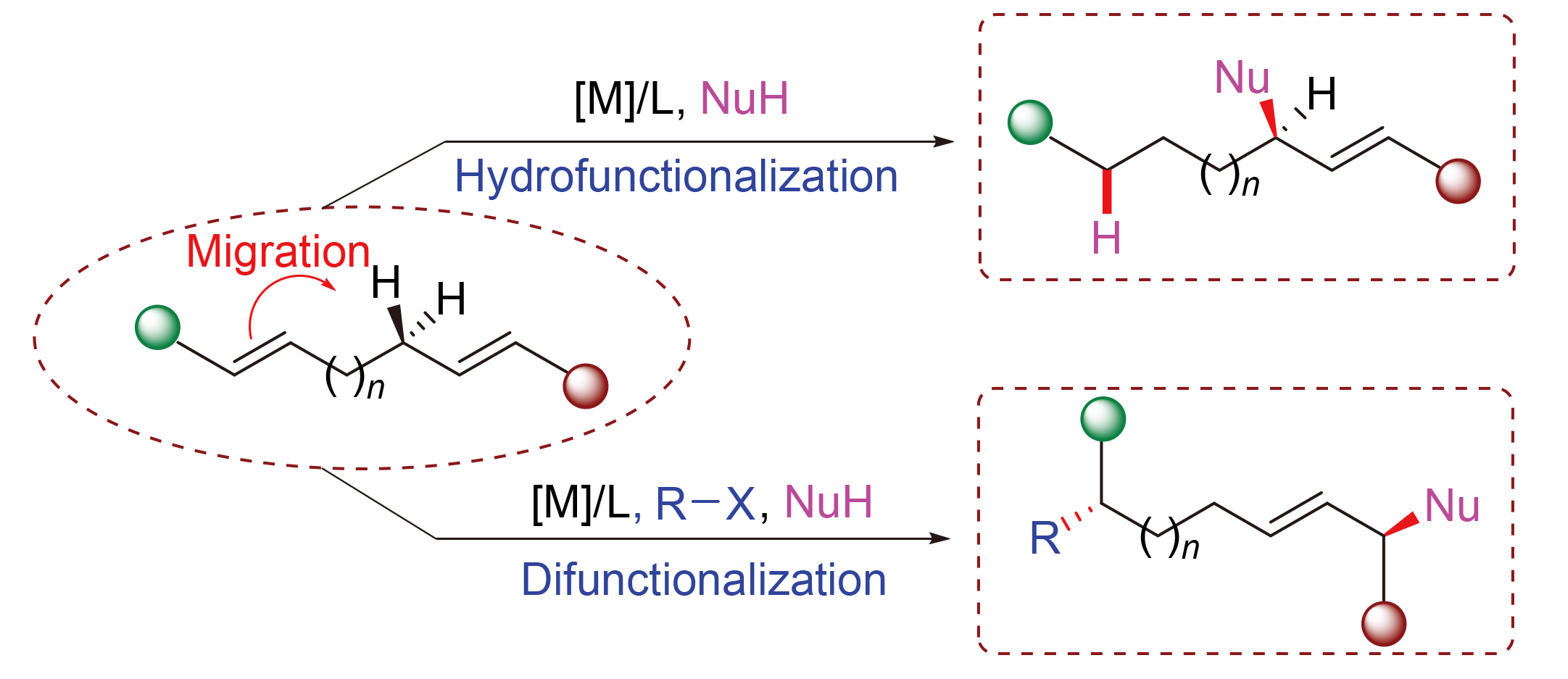
Asymmetric allylic C—H functionalization is a valuable and challenging research area. Different from the conventional direct allylic C—H cleavage strategy, transition metal-catalyzed migratory allylic substitution of remote dienes has emerged as a new route to achieve allylic C—H functionalization enantioselectively. This review provides a detailed summary of the development and advance of this strategy, introduces the related mechanistic processes, and discusses the area based on the types of catalysts and products.
| [1] | For selected reviews and work on typical transition metal-catalyzed asymmetric allylic substitution, see: (a) Trost, B. M.; Vranken Van, D. L. Chem. Rev. 1996, 96, 395. |
| [1] | (b) Trost, B. M.; Crawley, M. L. Chem. Rev. 2003, 103, 2921. |
| [1] | (c) Lu, Z.; Ma, S. Angew. Chem., Int. Ed. 2008, 47, 258. |
| [1] | (d) Trost, B. M. Tetrahedron 2015, 71, 5708. |
| [1] | (e) Butt, N. A.; Zhang, W. Chem. Soc. Rev. 2015, 44, 7929. |
| [1] | (f) Mohammadkhani, L.; Heravi, M. M. Chem. Rec. 2021, 21, 29. |
| [1] | (g) Süsse, L.; Stoltz, B. M. Chem. Rev. 2021, 121, 4084. |
| [1] | (h) Wang, R.-X.; Zhao, Q.-R.; Gu, Q.; You, S.-L. Acta Chim. Sinica 2023, 81, 431 (in Chinese). |
| [1] | (王瑞祥, 赵庆如, 顾庆, 游书力, 化学学报, 2023, 81, 431.) |
| [2] | For recent cases with C—C bond as leaving group, see: (a) Chen, Y.-W.; Qiu, Y.; Liu, Y.; Lin, G.-Q.; Hartwig, J. F. He, Z.-T. Nat. Synth. 2024, 3, 1011. |
| [2] | (b) Liu, Y.; Chen, Y.-W.; Yang, Y.-X.; Hartwig, J. F.; He, Z.-T. J. Am. Chem. Soc. 2024, 146, 29857. |
| [3] | For selected reviews, books and highlight on allylic C—H bond functionalization, see: (a) Jensen, T.; Fristrup, P. Chem.-Eur. J. 2009, 15, 9632. |
| [3] | (b) Liu, G.-S.; Wu, Y.-C. Activation, Springer, Berlin, Heidelberg, 2010, pp. 195-209. |
| [3] | (c) Liron, F.; Oble, J.; Lorion, M. M.; Poli, G. Eur. J. Org. Chem. 2014, 27, 5863. |
| [3] | (d) Tang, H.; Huo, X.; Meng, Q.; Zhang, W. Acta Chim. Sinica 2016, 74, 219 (in Chinese). |
| [3] | (汤淏溟, 霍小红, 孟庆华, 张万斌, 化学学报, 2016, 74, 219.) |
| [3] | (e) Wang, R.-H.; Luan, Y.-X.; Ye, M.-C. Chin. J. Chem. 2019, 37, 720. |
| [3] | (f) Wang, P.-S.; Gong, L.-Z. Acc. Chem. Res. 2020, 53, 2841. |
| [3] | (g) Yue, H.-F.; Zhu, C.; Huang, L.; Dewanjib, A.; Rueping, M. Chem. Commun. 2022, 58, 171. |
| [4] | (a) Li, J.-Y.; Zhang, Z.-H.; Wu, L.-Q.; Zhang, W.; Chen, P.-H.; Lin, Z.-Y.; Liu, G.-S. Nature 2019, 574, 516. |
| [4] | (b) Cheung, K. P. S.; Fang, J.; Mukherjee, K.; Mihranyan, A.; Gevorgyan, V. Science 2022, 378, 1207. |
| [5] | For selected reviews on metal-walking functionalization, see: (a) Vilches-Herrera, M.; Domke, L.; B?rner, A. ACS Catal. 2014, 4, 1706. |
| [5] | (b) Larionov, E.; Li, H.; Mazet, C. Chem. Commun. 2014, 50, 9816. |
| [5] | (c) Vasseur, A.; Bruffaerts, J.; Marek, I. Nat. Chem. 2016, 8, 209. |
| [5] | (d) Sommer, H.; Juli-Hernndez, F.; Martin, R.; Marek, I. ACS Cent. Sci. 2018, 4, 153. |
| [5] | (e) Kochi, T.; Kanno, S.; Kakiuchi, F. Tetrahedron Lett. 2019, 60, 150938. |
| [5] | (f) Massad, I.; Marek, I. ACS Catal. 2020, 10, 5793. |
| [5] | (g) Li, Y.; Wu, D.; Cheng, H.-G.; Yin, G.-Y. Angew. Chem., Int. Ed. 2020, 59, 7990. |
| [5] | (h) Janssen-Mller, D.; Sahoo, B.; Sun, S.-Z.; Martin, R. Isr. J. Chem. 2020, 60, 195. |
| [6] | For selected reviews on Pd-catalyzed enantioselective hydrofunctionalization of unsaturated bonds, see: (b) Haydl, A. M.; Breit, B.; Liang, T.; Krische, M. J. Angew. Chem., Int. Ed. 2017, 56, 11312. |
| [6] | (b) Li, G.; Huo, X.; Jiang, X.; Zhang, W. Chem. Soc. Rev. 2020, 49, 2060. |
| [6] | (c) Adamson, N. J.; Malcolmson, S. J. ACS Catal. 2020, 10, 1060. |
| [6] | (d) Blieck, R.; Taillefer, M.; Monnier, F. Chem. Rev. 2020, 120, 13545. |
| [6] | (e) Flaget, A.; Zhang, C.; Mazet, C. ACS Catal. 2022, 12, 15638. |
| [6] | (f) Cera, G.; Maestri, G. ChemCatChem 2022, 14, e202200295. |
| [6] | (g) Ma, C.; Chen, Y.-W.; He, Z.-T. Sci. Sin. Chim. 2023, 53, 474. |
| [6] | (h) Wang, Y.-C.; Liu, J.-B.; He, Z.-T. Chin. J. Org. Chem. 2023, 43, 2614 (in Chinese). |
| [6] | (王玉超, 刘晋彪, 何智涛, 有机化学, 2023, 43, 2614.) |
| [7] | For selected work on Pd-catalyzed enantioselective hydrofunctionalization of unsaturated bonds, see: (a) L?ber, O.; Kawatsura, M.; Hartwig, J. F. J. Am. Chem. Soc. 2001, 123, 4366. |
| [7] | (b) Leitner, A.; Larsen, J.; Steffens, C.; Hartwig, J. F. J. Org. Chem. 2004, 69, 7552. |
| [7] | (c) Zhou, H.; Wang, Y.-N.; Zhang, L.; Cai, M.; Luo, S.-Z. J. Am. Chem. Soc. 2017, 139, 3631. |
| [7] | (d) Adamson, N. J.; Hull, E.; Malcolmson, S. J. J. Am. Chem. Soc. 2017, 139, 7180. |
| [7] | (e) Adamson, N. J.; E. Wilbur, K. C.; Malcolmson, S. J. J. Am. Chem. Soc. 2018, 140, 2761. |
| [7] | (f) Nie, S.-Z.; Davison, R. T.; Dong, V. M. J. Am. Chem. Soc. 2018, 140, 16450. |
| [7] | (g) Park, S.; Malcolmson, S. J. ACS Catal. 2018, 8, 8468. |
| [7] | (h) Su, Y.-L.; Li, L.-L.; Zhou, X.-L.; Dai, Z.-Y.; Wang, P.-S.; Gong, L.-Z. Org. Lett. 2018, 20, 2403. |
| [7] | (i) Zhang, Q.-L.; Yu, H.-M.; Shen, L.-L.; Tang, T.-H.; Dong, D.-F.; Chai, W.-W.; Zi, W.-W. J. Am. Chem. Soc. 2019, 141, 14554. |
| [7] | (j) Park, S.; Adamson, N. J.; Malcolmson, S. J. Chem. Sci. 2019, 10, 5176. |
| [7] | (k) Zhang, Z.; Xiao, F.; Wu, H.-M.; Dong, X.-Q.; Wang, C.-J. Org. Lett. 2020, 22, 569. |
| [7] | (l) Onyeagusi, C. I.; Shao, X.; Malcolmson, S. J. Org. Lett. 2020, 22, 1681. |
| [7] | (m) Adamson, N. J.; Park, S.; Zhou, P.; Nguyen, A. L.; Malcolmson, S. J. Org. Lett. 2020, 22, 2032. |
| [7] | (n) Yang, H.; Xing, D. Chem. Commun. 2020, 56, 3721. |
| [7] | (o) Zhang, Q.; Dong, D.; Zi, W. J. Am. Chem. Soc. 2020, 142, 15860. |
| [7] | (p) Li, M.-M.; Cheng, L.; Xiao, L.-J.; Xie, J.-H.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2021, 60, 2948. |
| [7] | (q) Yang, S.-Q.; Wang, Y.-F.; Zhao, W.-C.; Lin, G.-Q.; He, Z.-T. J. Am. Chem. Soc. 2021, 143, 7285. |
| [7] | (r) Wang, H.; Zhang, R.; Zhang, Q.; Zi, W. J. Am. Chem. Soc. 2021, 143, 10948. |
| [7] | (s) Jiu, A. Y.; Slocumb, H. S.; Yeung, C. S.; Yang, X.-H.; Dong, V. M. Angew. Chem., Int. Ed. 2021, 60, 19660. |
| [7] | (t) Zhang, Q.; Zhu, M.; Zi, W. Chem 2022, 8, 2784. |
| [7] | (u) Yang, S.-Q.; Han, A.-J.; Liu, Y.; Tang, X.-Y.; Lin, G.-Q.; He, Z.-T. J. Am. Chem. Soc. 2023, 145, 3915. |
| [7] | (v) Tang, M.-Q.; Yang, Z.-J.; He, Z.-T. Nat. Commun. 2023, 14, 6303. |
| [7] | (w) Han, A.-J.; Tan, Q.-T.; He, Z.-T. Org. Lett. 2024, 26, 89. |
| [7] | (x) Yang, Z.-J.; He, Z.-T. Synthesis 2024, 56, 3412. |
| [7] | (y) Zhang, J.-M.; Wang, Y.-C.; Chen, L.; Ma, C.; He, Z.-T. Chem.- Eur. J. 2024, 30, e202401350. |
| [7] | (z) Xie, B.-Y.; He, Z.-T. ACS Catal. 2024, 14, 9742. |
| [8] | Chen, Y.-W.; Liu, Y.; Lu, H.-Y.; Lin, G.-Q.; He, Z.-T. Nat. Commun. 2021, 12, 5626. |
| [9] | Liao, Q.-Y.; Ma, C.; Wang, Y.-C. Yang, S.-Q.; Ma, J.-S.; He, Z.-T. Chin. Chem. Lett. 2023, 34, 108371. |
| [10] | Wang, Y. -C, Xiao. Z.-X.; Wang, M.; Yang, S.-Q.; Liu, J.-B.; He, Z.-T. Angew. Chem., Int. Ed. 2023, 62, e202215568. |
| [11] | For selected examples on Pd-catalyzed asymmetric metal walking, see: (a) Werner, E.W.; Mei, T.-S.; Burckle, M.; Sigman, M. S. Science 2012, 338, 1455. |
| [11] | (b) Mei, T.-S.; Patel, H. H.; Sigman, M. S. Nature 2014, 508, 340. |
| [11] | (c) Larionov, E.; Lin, L.; Guénée, L.; Mazet, C. J. Am. Chem. Soc. 2014, 136, 16882. |
| [11] | (d) He, Y.; Yang, Z.; Thornbury, R. T.; Toste, F. D. J. Am. Chem. Soc. 2015, 137, 12207. |
| [11] | (e) Nelson, H. M.; Williams, B. D.; Miró, J.; Toste, F. D. J. Am. Chem. Soc. 2015, 137, 3213. |
| [11] | (f) Lin, L.; Romano, C.; Mazet, C. J. Am. Chem. Soc. 2016, 138, 10344. |
| [11] | (g) Kou, X.; Shao, Q.; Ye, C.; Yang, G.; Zhang, W. J. Am. Chem. Soc. 2018, 140, 7587. |
| [11] | (h) Liu, J.; Yuan, Q.; Toste, F. D.; Sigman, M. S. Nat. Chem. 2019, 11, 710. |
| [11] | (i) Li, X.; Yang, T.; Li, J.; Li, X.; Chen, P.; Lin, Z.; Liu, G. Nat. Chem. 2023, 15, 862. |
| [12] | Chen, X.-X.; Luo, H.; Chen, Y.-W.; Liu, Y.; He, Z.-T. Angew. Chem., Int. Ed. 2023, 62, e202307628. |
| [13] | Miao, H.-Z.; Liu, Y.; Chen, Y.-W.; Lu, H.-Y.; Li, J.; Lin, G.-Q.; He, Z.-T. Synlett 2023, 34, 451. |
| [14] | Li, G.-L.; Huo, X.-H.; Jiang, X.-Y.; Zhang, W.-B. Chem. Soc. Rev. 2020, 49, 2060. |
| [15] | Bender, D. D.; Stakem, F. G.; Heck, R. F. J. Org. Chem. 1982, 47, 1278. |
| [16] | Larock, R. C.; Lu, Y.-D.; Bain, A. C. J. Org. Chem. 1991, 56, 4589. |
| [17] | (a) Larock, R. C.; Berrios-Pefia, N. G.; Fried, C. A.; Yum, E. K.; Tu, C.; Leong, W. J. Org. Chem. 1993, 58, 4510. |
| [17] | (b) Larock, R. C.; Wang, Yao.; Lu, Y.-D.; Russell, C. E. J. Org. Chem. 1994, 59, 8107. |
| [17] | (c) Wang, Y.; Dong, X.-Y.; Larock, R. C. J. Org. Chem. 2003, 68, 3091. |
| [18] | Pang, H.-L.; Wu, D.; Cong, H.-J.; Yin, G.-Y. ACS Catal. 2019, 9, 8555. |
| [19] | Pang, H.-L.; Wu, D.; Yin, G.-Y. Chin. J. Org. Chem. 2021, 41, 849 (in Chinese). |
| [19] | (庞海亮, 吴冬, 阴国印, 有机化学, 2021, 41, 849.) |
| [20] | Zhu, D.; Jiao, Z.; Chi, Y. R.; Goncalves, T. P.; Huang, K.-W.; Zhou, J. S. Angew. Chem., Int. Ed. 2020, 59, 5341. |
| [21] | Zhu, D.-Y.; Xu, W.-Q.; Pu, M.-P.; Wu, Y.-D.; Chi, Y. R.; Zhou, J. S. Org. Lett. 2021, 23, 7064. |
| [22] | Han, X.-J.; Larock, R. C. Synlett 1998, 7, 748. |
| [23] | Zhang, Y.; Shen, H.-C.; Li, Y.-Y.; Huang, Y.-S.; Han, Z.-Y.; Wu, X. Chem. Commun. 2019, 55, 3769. |
| [24] | Lux, M. C.; Boby, M. L.; Brooksc, J. L.; Tan, D. S. Chem. Commun. 2019, 55, 7013. |
| [25] | Yu, R.-R.; Rajasekar, S.; Fang, X.-J. Angew. Chem., Int. Ed. 2020, 59, 21436. |
| [26] | Cao, Y.-X.; Wodrich, M. D.; Cramer, D. Nat. Commun. 2023, 14, 7640. |
| [27] | Wang, X.; Miao, H.-Z.; Lin, G.-Q.; He, Z.-T. Angew. Chem., Int. Ed. 2023, 62, e202301556. |
/
| 〈 |
|
〉 |