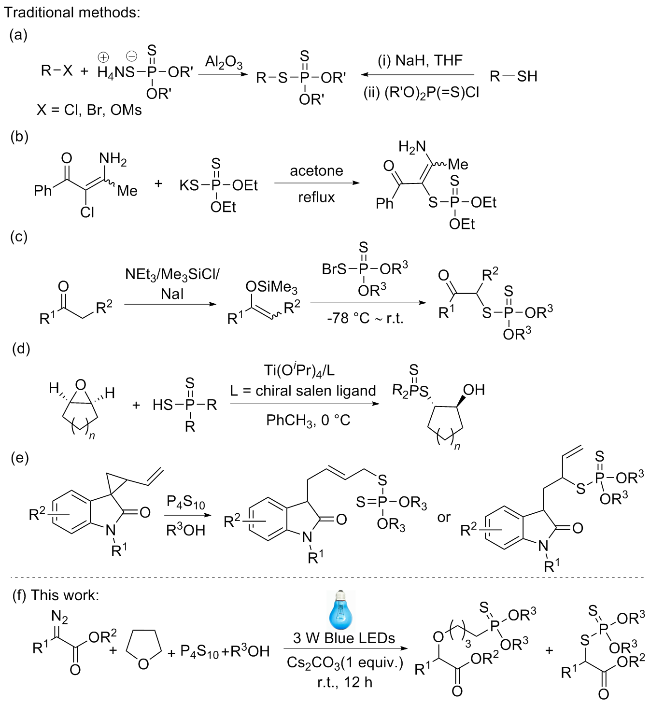
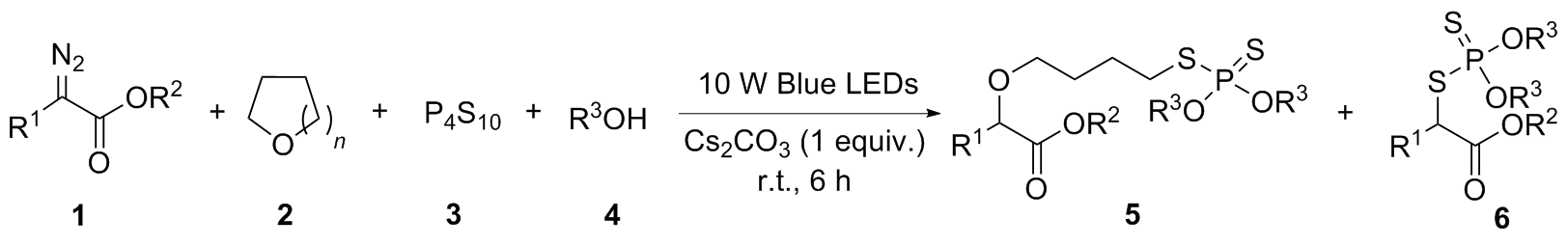在室温条件下, 向10 mL反应管中加入P4S10 (0.2 mmol)、Cs2CO3 (0.2 mmol)和乙醇(0.5 mL), 再加入α-重氮酯(0.2 mmol), 用四氢呋喃(1.5 mL)润洗反应管内壁. 然后将反应管置于10 W蓝光光反应器下, 空气中搅拌6 h. 用薄层色谱(TLC)检测反应的完成情况, 当原料反应完成后, 将反应液转移到50 mL圆底烧瓶中, 加入硅胶粉后减压蒸干溶剂. 然后用石油醚/乙酸乙酯(体积比为20∶1)柱层析分离得到烷基二硫代磷酸酯.
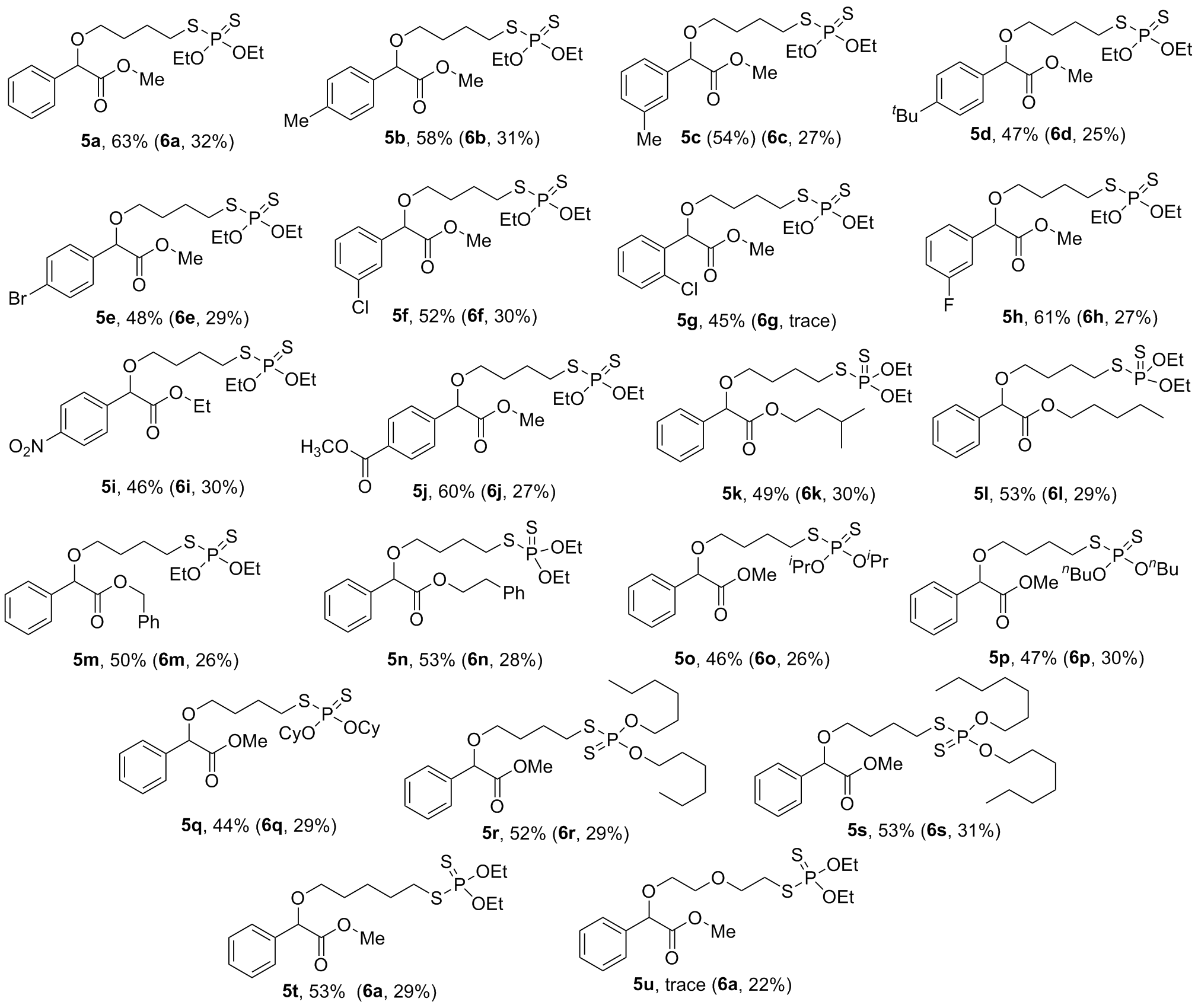
2-(4-(二乙氧基磷硫基)硫代)丁氧基)-2-苯乙酸甲酯(5a): 黄色油状51.3 mg, 产率63%. 1H NMR (500 MHz, CDCl3) δ: 7.42~7.44 (m, 2H), 7.32~7.37 (m, 3H), 4.85 (s, 1H), 4.02~4.23 (m, 4H), 3.70 (s, 3H), 3.53~3.58 (m, 1H), 3.43~3.47 (m, 1H), 2.86~2.93 (m, 2H), 1.72~1.83 (m, 4H), 1.34 (dt, J=7.1, 1.9 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.3, 136.5, 128.6, 128.6, 127.1, 81.1, 69.0, 63.8 (d, J=5.9 Hz), 52.2, 33.3 (d, J=4.0 Hz), 28.5, 27.1 (d, J=5.4 Hz), 15.8 (d, J=8.4 Hz); HRMS (ESI- TOP) calcd for C17H27O5PS2Na [M+Na]+ 429.0935, found 429.0931.
2-(4-(二乙氧基磷酸硫代)硫代)丁氧基)-2-(对甲苯基)乙酸甲酯(5b): 黄色油状48.8 mg, 产率58%. 1H NMR (500 MHz, CDCl3) δ: 7.31 (d, J=8.0 Hz, 2H), 7.16 (d, J=8.0 Hz, 2H), 4.81 (s, 1H), 4.08~4.21 (m, 4H), 3.69 (s, 3H), 3.51~3.55 (m, 1H), 3.41~3.45 (m, 1H), 2.85~2.91 (m, 2H), 2.34 (s, 3H), 1.71~1.80 (m, 4H), 1.34 (dt, J=7.1, 1.8 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.4, 138.5, 123.5, 129.3, 127.1, 80.9, 68.8, 63.8 (d, J=5.8 Hz), 52.1, 33.3 (d, J=4.0 Hz), 28.5, 27.1 (d, J=5.5 Hz), 21.2, 15.8 (d, J=8.3 Hz); HRMS (ESI-TOP) calcd for C18H29O5PS2Na [M+Na]+ 443.01092, found 443.1085.
2-(4-(二氧基硫基磷酸基)硫代)丁氧基)-2-(间甲苯基)乙酸甲酯(5c): 黄色油状45.4 mg, 产率54%. 1H NMR (500 MHz, CDCl3) δ: 7.20~7.24 (m, 3H), 7.13~7.14 (m, 1H), 4.81 (s, 1H), 4.08~4.21 (m, 4H), 3.70 (s, 3H), 3.52~3.56 (m, 1H), 3.42~3.46 (m, 1H), 2.86~2.92 (m, 2H), 2.35 (s, 3H), 1.71~1.83 (m, 4H), 1.34 (dt, J=7.1, 2.1 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.4, 138.4, 136.3, 129.4, 128.5, 127.7, 124.3, 81.1, 68.9, 63.8 (d, J=5.8 Hz), 52.2, 33.3 (d, J=4.0 Hz), 28.5, 27.1 (d, J=5.4 Hz), 21.4, 15.8 (d, J=8.3 Hz); HRMS (ESI-TOP) calcd for C18H29O5PS2Na [M+Na]+ 443.1092, found 443.1081.
2-(4-(叔丁基)苯基)-2-(4-(二乙氧基磷酸硫基)硫代)丁氧基)乙酸甲酯(5d): 黄色油状43.5 mg, 产率47%. 1H NMR (500 MHz, CDCl3) δ: 7.33~7.48 (m, 4H), 4.83 (s, 3H), 4.08~4.23 (m, 4H), 3.70 (s, 3H), 3.52~3.56 (m, 1H), 3.43~3.47 (m, 1H), 2.86~2.92 (m, 2H), 1.72~1.81 (m, 4H), 1.34 (dt, J=7.1, 2.1 Hz, 6H), 1.37 (s, 9H); 13C NMR (125 MHz, CDCl3) δ: 171.5, 151.6, 133.4, 126.8, 125.5, 80.9, 68.9, 63.8 (d, J=5.8 Hz), 52.1, 34.6, 33.3 (d, J=4.0 Hz), 31.2, 28.5, 27.1 (d, J=5.5 Hz), 15.8 (d, J=8.3 Hz); HRMS (ESI-TOP) calcd for C21H35- O5PS2Na [M+Na]+ 485.1561, found 485.1561.
2-(4-溴苯基)-2-(4-(二氧基硫基磷酸基)硫代)丁氧基)乙酸甲酯(5e): 黄色油状46.6 mg, 产率48%. 1H NMR (500 MHz, CDCl3) δ: 7.49 (d, J=8.5 Hz, 2H), 7.32 (d, J=8.4 Hz, 2H), 4.81 (s, 1H), 4.08~4.22 (m, 4H), 3.70 (s, 3H), 3.54~3.58 (m, 1H), 3.42~3.46 (m, 1H), 2.86~2.92 (m, 2H), 1.73~1.81 (m, 4H), 1.34 (dt, J=7.1 Hz, J2=2.1 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.8, 135.5, 131.7, 128.7, 122.7, 80.4, 69.2, 63.8 (d, J=6.0 Hz), 52.3, 33.3 (d, J=3.9 Hz), 28.4, 27.1 (d, J=5.4 Hz), 15.8 (d, J=8.3 Hz); 31P NMR (202 MHz, CDCl3) δ: 95.1; HRMS (ESI-TOP) calcd for C17H26BrO5PS2Na [M+Na]+ 507.0040, found 507.0023.
2-(3-氯苯基)-2-(4-(二乙氧基磷酸硫基)硫代)丁氧基)乙酸甲酯(5f): 黄色油状45.8 mg, 产率52%. 1H NMR (500 MHz, CDCl3) δ: 7.43 (s, 1H), 7.28~7.32 (m, 3H), 7.32~7.37 (m, 3H), 4.02~4.23 (m, 4H), 3.70 (s, 3H), 3.55~3.57 (m, 1H), 3.44~3.47 (m, 1H), 2.87~2.93 (m, 2H), 1.74~1.81 (m, 4H), 1.34 (t, J=7.1 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.7, 138.4, 124.5, 129.8, 128.8, 127.2, 125.2, 80.4, 69.3, 63.9 (d, J=6.0 Hz), 52.4, 33.3 (d, J=3.9 Hz), 28.4, 27.1 (d, J=5.4 Hz), 15.8 (d, J=8.3 Hz); HRMS (ESI-TOP) calcd for C17H26ClO5PS2Na [M+Na]+ 463.0545, found 463.0536.
2-(2-氯苯基)-2-(4-(二氧基硫基磷)硫代)丁氧基)乙酸甲酯(5g): 黄色油状39.7 mg, 产率45%. 1H NMR (500 MHz, CDCl3) δ: 7.48~7.50 (m, 1H), 7.37~7.39 (m, 1H), 7.26~7.29 (m, 2H), 5.33 (s, 1H), 4.08~4.20 (m, 4H), 3.72 (s, 3H), 3.60~3.64 (m, 1H), 3.45~3.49 (m, 1H), 2.85~2.91 (m, 2H), 1.72~1.81 (m, 4H), 1.34 (t, J=7.1 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.6, 134.5, 133.7, 129.8, 129.6, 128.7, 127.2, 77.2, 69.4, 63.8 (d, J=5.9 Hz), 52.3, 33.3 (d, J=4.0 Hz), 28.5, 27.0 (d, J=5.5 Hz), 15.8 (d, J=8.4 Hz); HRMS (ESI-TOP) calcd for C17- H26ClO5PS2Na [M+Na]+ 463.0545, found 463.0537.
2-(4-(二氧基磷硫基)硫代)丁氧基)-2-(3-氟苯基)乙酸甲酯(5h): 黄色油状51.8 mg, 产率61%. 1H NMR (500 MHz, CDCl3) δ: 7.30~7.34 (m, 1H), 7.20~7.24 (m, 1H), 7.16~7.18 (m, 1H), 7.00~7.04 (m, 1H), 4.85 (s, 1H), 4.08~4.22 (m, 4H), 3.72 (s, 3H), 3.55~3.59 (m, 1H), 3.45~3.48 (m, 1H), 2.87~2.93 (m, 2H), 1.74~1.81 (m, 4H), 1.35 (t, J=7.1 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.8, 162.8 (d, J=245.4 Hz), 138.9 (d, J=7.3 Hz), 130.1 (d, J=8.0 Hz), 122.7 (d, J=3.0 Hz), 115.6 (d, J=21.0 Hz), 114.1 (d, J=22.5 Hz), 80.4, 69.2, 63.9 (d, J=6.2 Hz), 52.3, 33.3 (d, J=4.0 Hz), 28.5, 27.1 (d, J=5.3 Hz), 15.8 (d, J=8.3 Hz); HRMS (ESI-TOP) calcd for C17H26- FO5PS2Na [M+Na]+ 447.0841, found 447.0839.
2-(4-(二氧基磷酸硫代)硫代)丁氧基)-2-(4-硝基苯基)乙酸乙酯(5i): 黄色油状42.8 mg, 产率46%. 1H NMR (500 MHz, CDCl3) δ: 8.15 (d, J=8.6 Hz, 2H), 7.58 (d, J=8.7 Hz, 2H), 4.87 (s, 1H), 4.04~4.15 (m, 6H), 3.70 (s, 3H), 3.56~3.60 (m, 1H), 3.40~3.43 (m, 1H), 2.82~2.88 (m, 2H), 1.70~1.77 (m, 4H), 1.27 (dt, J=7.1, 2.6 Hz, 6H), 1.15 (t, J=7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 169.7, 148.0, 143.6, 127.7, 123.7, 80.2, 69.6, 63.9 (d, J=6.1 Hz), 61.7, 33.3 (d, J=3.9 Hz), 28.4, 27.1 (d, J=5.5 Hz), 15.8 (d, J=8.2 Hz), 14.0; HRMS (ESI-TOP) calcd for C18H28NO7PS2Na [M+Na]+ 488.0942, found 488.0941.
4-(1-(4-(二乙氧基磷硫基)硫代)丁氧基)-2-甲氧基- 2-氧代乙基)苯甲酸甲酯(5j): 黄色油状55.8 mg, 产率60%. 1H NMR (500 MHz, CDCl3) δ: 8.03 (d, J=8.3 Hz, 2H), 7.52 (d, J=8.3 Hz, 2H), 4.91 (s, 1H), 4.08~4.23 (m, 4H), 3.91 (s, 3H), 3.71 (s, 3H), 3.56~3.61 (m, 1H), 3.44~3.48 (m, 1H), 2.87~2.93 (m, 2H), 1.75~1.83 (m, 4H), 1.34 (dt, J=7.1, 2.3 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.7, 176.6, 141.3, 130.4, 129.8, 127.0, 80.6, 69.3, 63.8 (d, J=6.0 Hz), 52.4, 52.1, 33.3 (d, J=3.9 Hz), 28.4, 27.1 (d, J=5.4 Hz), 21.2, 15.8 (d, J=8.3 Hz); HRMS (ESI- TOP) calcd for C19H29O7PS2Na [M+Na]+ 487.0990, found 487.0995.
异戊基2-(4-(二氧基磷硫代)硫代)丁氧基)-2-苯乙酸酯(5k): 黄色油状45.4 mg, 产率49%. 1H NMR (500 MHz, CDCl3) δ: 7.43~7.45 (m, 2H), 7.31~7.36 (m, 3H), 4.84 (s, 1H), 4.08~4.21 (m, 4H), 3.85~3.92 (m, 2H), 3.55~3.60 (m, 1H), 3.44~3.48 (m, 1H), 2.86~2.92 (m, 2H), 1.85~1.89 (m, 1H), 1.74~1.84 (m, 4H), 1.34 (dt, J=7.1, 1.4 Hz, 6H), 0.82 (dd, J=6.7, 1.4 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.9, 136.7, 128.5, 128.5, 127.1, 81.1, 71.0, 69.0, 63.8 (d, J=5.9 Hz), 33.3 (d, J=3.9 Hz), 28.5, 27.6, 27.1 (d, J=5.4 Hz), 18.8, 15.8 (d, J=8.3 Hz); HRMS (ESI-TOP) calcd for C21H35O5PS2Na [M+Na]+ 485.1561, found 485.1552.
正戊基2-(4-(二氧基磷硫基)硫代)丁氧基)-2-苯乙酸酯(5l): 黄色油状49.1 mg, 产率53%. 1H NMR (500 MHz, CDCl3) δ: 7.43~7.44 (m, 2H), 7.32~7.37 (m, 3H), 4.85 (s, 1H), 4.02~4.23 (m, 4H), 3.70 (s, 3H), 3.53~3.58 (m, 1H), 3.43~3.47 (m, 1H), 2.86~2.93 (m, 2H), 1.72~1.83 (m, 4H), 1.34 (dt, J=7.1, 1.9 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.3, 136.5, 128.6, 128.6, 127.1, 81.1, 69.0, 63.8 (d, J=5.9 Hz), 52.2, 33.3 (d, J=4.0 Hz), 28.5, 27.1 (d, J=5.4 Hz), 15.8 (d, J=8.4 Hz); HRMS (ESI- TOP) calcd for C21H35O5PS2Na [M+Na]+ 485.1561, found 485.1551.
苄基2-(4-(二氧基磷硫基)硫代)丁氧基)-2-苯乙酸酯(5m): 黄色油状48.3 mg, 产率50%. 1H NMR (500 MHz, CDCl3) δ: 7.42~7.44 (m, 2H), 7.31~7.36 (m, 3H), 4.83 (s, 1H), 4.08~4.19 (m, 6H), 3.55~3.59 (m, 1H), 3.45~3.48 (m, 1H), 2.86~2.92 (m, 2H), 1.75~1.81 (m, 4H), 1.53~1.59 (m, 2H), 1.34 (dt, J=7.1, 1.7 Hz, 6H), 1.17~1.25 (m, 4H), 0.83 (t, J=7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 170.9, 136.7, 128.5, 128.5, 127.1, 81.1, 68.9, 65.2, 63.8 (d, J=5.9 Hz), 33.3 (d, J=4.0 Hz), 28.5, 28.1, 27.8, 27.1 (d, J=5.5 Hz), 22.1, 15.8 (d, J=8.3 Hz), 13.8; HRMS (ESI-TOP) calcd for C23H31O5PS2Na [M+Na]+ 505.1248, found 505.1243.
苯乙基2-(4-((二乙氧基磷酸硫基)硫代)丁氧基)-2-苯乙酸酯(5n): 黄色油状52.7 mg, 产率53%. 1H NMR (500 MHz, CDCl3) δ: 7.37~7.39 (m, 2H), 7.32~7.34 (m, 3H), 7.19~7.25 (m, 3H), 7.06~7.08 (m, 2H), 4.80 (s, 1H), 4.30~4.34 (m, 2H), 4.08~4.20 (m, 4H), 3.48~3.52 (m, 1H), 3.38~3.42 (m, 1H), 2.85~2.91 (m, 2H), 1.70~1.79 (m, 4H), 1.33 (dt, J=7.1, 1.0 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.8, 137.4, 136.5, 128.8, 128.6, 128.5, 128.4, 127.1, 126.5, 81.1, 68.9, 66.5, 63.8 (d, J=5.9 Hz), 34.9, 33.3 (d, J=4.0 Hz), 28.5, 27.1 (d, J=5.4 Hz), 15.8 (d, J=8.3 Hz); HRMS (ESI-TOP) calcd for C24H33O5- PS2Na [M+Na]+ 519.1405, found 519.1400.
2-(4-(二异丙氧基磷硫酰基)硫代)丁氧基)-2-苯乙酸甲酯(5o): 黄色油状40.0 mg, 产率46%. 1H NMR (500 MHz, CDCl3) δ: 7.42~7.44 (m, 2H), 7.32~7.37 (m, 3H), 4.85 (s, 1H), 4.78~4.83 (m, 4H), 3.70 (s, 3H), 3.53~3.57 (m, 1H), 3.43~3.47 (m, 1H), 2.88~2.93 (m, 2H), 1.73~1.82 (m, 4H), 1.31~1.34 (m, 12H); 13C NMR (125 MHz, CDCl3) δ: 171.3, 136.5, 128.6, 128.6, 127.1, 81.1, 73.3 (d, J=6.8 Hz), 69.0, 52.2, 33.4 (d, J=3.8 Hz), 28.5, 26.9 (d, J=6.2 Hz), 23.7 (d, J=4.5 Hz), 23.4 (d, J=5.1 Hz); HRMS (ESI-TOP) calcd for C19H31O5PS2Na [M+Na]+ 457.1248, found 457.1243.
2-(4-(二丁氧基磷硫基)硫代)丁氧基)-2-苯乙酸甲酯(5p): 黄色油状43.5 mg, 产率47%. 1H NMR (500 MHz, CDCl3) δ: 7.42~7.44 (m, 2H), 7.32~7.37 (m, 3H), 4.85 (s, 1H), 4.01~4.13 (m, 4H), 3.70 (s, 3H), 3.53~3.56 (m, 1H), 3.44~3.47 (m, 1H), 2.85~2.91 (m, 2H), 1.74~1.80 (m, 4H), 1.64~1.68 (m, 4H), 1.38~1.42 (m, 4H), 0.93 (t, J=7.4 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.3, 136.5, 128.6, 128.6, 127.1, 81.0, 69.0, 67.6 (d, J=6.5 Hz), 52.2, 33.3 (d, J=4.0 Hz), 32.0 (d, J=8.2 Hz), 28.5, 27.1 (d, J=5.5 Hz), 18.8, 13.6; HRMS (ESI-TOP) calcd for C21H35O5PS2Na [M+Na]+ 485.1561, found 458.1552.
2-(4-((双(环己氧基)磷酸硫代)硫代)丁氧基)-2-苯乙酸甲酯(5q): 黄色油状45.3 mg, 产率44%. 1H NMR (500 MHz, CDCl3) δ: 7.42~7.44 (m, 2H), 7.32~7.37 (m, 3H), 4.85 (s, 1H), 4.51~4.57 (m, 2H), 3.70 (s, 3H), 3.54~3.56 (m, 1H), 3.43~3.46 (m, 1H), 2.88~2.94 (m, 2H), 1.93 (s, 4H), 1.71~1.82 (m, 8H), 1.48~1.56 (m, 6H), 1.31~1.38 (m, 4H), 1.23~1.27 (m, 2H); 13C NMR (125 MHz, CDCl3) δ: 171.3, 136.5, 128.6, 128.6, 127.1, 81.0, 78.1 (d, J=7.5 Hz), 69.1, 52.2, 33.4 (d, J=3.8 Hz), 33.1 (d, J=4.6 Hz), 28.5, 26.9 (d, J=6.1 Hz), 23.1, 23.7 (d, J=5.2 Hz); HRMS (ESI-TOP) calcd for C25H39O5PS2Na [M+Na]+ 537.1874, found 537.1870.
2-(4-(双(己氧基)磷酸硫代)硫代)丁氧基)-2-苯乙酸甲酯(5r): 黄色油状54.0 mg, 产率52%. 1H NMR (500 MHz, CDCl3) δ: 7.42~7.44 (m, 2H), 7.32~7.37 (m, 3H), 4.85 (s, 1H), 3.99~4.14 (m, 4H), 3.70 (s, 3H), 3.53~3.57 (m, 1H), 3.42~3.47 (m, 1H), 2.85~2.91 (m, 2H), 1.73~1.81 (m, 4H), 1.65~1.71 (m, 4H), 1.25~1.38 (m, 12H), 0.88 (t, J=6.7 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.3, 136.5, 128.6, 128.6, 127.1, 81.1, 69.0, 67.9 (d, J=6.6 Hz), 52.2, 33.3, 31.3, 29.9 (d, J=8.1 Hz), 28.5, 27.1 (d, J=5.6 Hz), 25.2, 22.5, 13.9; HRMS (ESI-TOP) calcd for C25H43O5PS2Na [M+Na]+ 541.2187, found 541.2180.
2-(4-(双(庚氧基)磷酸硫代)硫代)丁氧基)-2-苯乙酸甲酯(5s): 黄色油状58.0 mg, 产率53%. 1H NMR (500 MHz, CDCl3) δ: 7.42~7.44 (m, 2H), 7.32~7.37 (m, 3H), 4.85 (s, 1H), 3.98~4.14 (m, 4H), 3.70 (s, 3H), 3.53~3.57 (m, 1H), 3.44~3.47 (m, 1H), 2.85~2.91 (m, 2H), 1.74~1.80 (m, 4H), 1.65~1.69 (m, 4H), 1.25~1.37 (m, 16H), 0.88 (t, J=6.7 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.3, 136.5, 128.6, 128.6, 127.1, 81.1, 69.0, 67.9 (d, J=6.6 Hz), 52.2, 33.3 (d, J=3.9 Hz), 31.7, 30.0 (d, J=8.1 Hz), 28.8, 28.5, 27.1 (d, J=5.6 Hz), 25.5, 22.5, 14.0; HRMS (ESI-TOP) calcd for C27H47O5PS2Na [M+Na]+ 569.2500, found 569.2496.
2-((5-(二氧基磷酸硫代)硫代)戊基)氧基)-2-苯乙酸甲酯(5t): 黄色油状44.6 mg, 产率53%. 1H NMR (500 MHz, CDCl3) δ: 7.42~7.44 (m, 2H), 7.31~7.38 (m, 3H), 4.85 (s, 1H), 4.08~4.22 (m, 4H), 3.70 (s, 3H), 3.51~3.55 (m, 1H), 3.40~3.45 (m, 1H), 2.82~2.88 (m, 2H), 1.64~1.71 (m, 4H), 1.45~1.51 (m, 2H), 1.34 (t, J=7.1 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 171.4, 136.6, 128.6, 128.6, 127.1, 81.1, 69.5, 63.8 (d, J=5.9 Hz), 52.2, 33.4 (d, J=4.0 Hz), 30.1 (d, J=5.5 Hz), 29.0, 25.2, 15.9 (d, J=8.4 Hz); 31P NMR (202 MHz, CDCl3) δ: 95.1; HRMS (ESI-TOP) calcd for C18H29O5PS2Na [M+Na]+ 443.1092, found 443.1082.
2-((二乙氧基硫基磷酸基)硫代)-2-苯乙酸甲酯(6a): 黄色油状21.6 mg, 产率31%. 1H NMR (500 MHz, CDCl3) δ: 7.43~7.45 (m, 2H), 7.30~7.35 (m, 3H), 5.02 (d, J=13.1 Hz, 1H), 3.89~4.18 (m, 4H), 3.73 (s, 3H), 1.21~1.27 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.2 (d, J=6.4 Hz), 135.9 (d, J=4.8 Hz), 128.8, 128.6, 128.4, 64.2 (d, J=5.1 Hz), 54.4 (d, J=2.8 Hz), 53.1, 15.6 (dd, J=8.7, 3.6 Hz); HRMS (ESI-TOP) calcd for C13H19O4PS2 [M+H]+ 335.0535, found 335.0537.
2-(二氧基磷硫代)硫代)-2-(对甲苯基)乙酸甲酯(6b): 黄色油状21.5 mg, 产率32%. 1H NMR (500 MHz, CDCl3) δ: 7.32 (d, J=8.0 Hz, 2H), 7.14 (d, J=7.9 Hz, 2H), 5.01 (d, J=13.0 Hz, 1H), 3.91~4.18 (m, 4H), 3.72 (s, 3H), 2.33 (s, 3H), 1.23~1.27 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.4 (d, J=5.9 Hz), 138.5, 132.8 (d, J=5.4 Hz), 130.2, 129.6, 129.5, 128.2, 64.2 (d, J=5.0 Hz), 54.4 (d, J=2.9 Hz), 53.0, 21.4, 15.6 (dd, J=8.7, 1.8 Hz); HRMS (ESI-TOP) calcd for C14H21O4PS2Na [M+Na]+ 371.0517, found 371.0505.
2-(二氧基硫基磷酰基)硫代)-2-(间甲苯基)乙酸甲酯 (6c): 黄色油状18.9 mg, 产率27%. 1H NMR (500 MHz, CDCl3) δ: 7.20~7.24 (m, 3H), 7.11~7.12 (m, 1H), 5.00 (d, J=13.1 Hz, 1H), 3.90~4.18 (m, 4H), 3.73 (s, 3H), 2.34 (s, 3H), 1.21~1.27 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.3 (d, J=6.0 Hz), 138.6, 135.7 (d, J=5.3 Hz), 129.3, 128.9, 128.7, 125.4, 64.2 (dd, J=5.4, 3.6 Hz), 54.4 (d, J=2.8 Hz), 53.1, 12.3, 15.6 (dd, J=8.7, 4.9 Hz); HRMS (ESI-TOP) calcd for C14H22O4PS2 [M+H]+ 349.0697, found 349.0693.
2-(4-(叔丁基)苯基)-2-(二氧基硫基磷基)硫代)乙酸甲酯(6d): 黄色油状19.6 mg, 产率25%. 1H NMR (500 MHz, CDCl3) δ: 7.34~7.37 (m, 4H), 5.00 (d, J=12.8 Hz, 1H), 3.86~4.19 (m, 4H), 3.73 (s, 3H), 1.29 (s, 9H), 1.20~1.26 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.3 (d, J=6.4 Hz), 151.7, 132.8 (d, J=5.1 Hz), 128.0, 125.8, 64.2 (dd, J=5.4, 2.7 Hz), 54.1 (d, J=2.8 Hz), 53.0, 34.6, 31.2, 15.6 (dd, J=8.7, 5.0 Hz); HRMS (ESI-TOP) calcd for C17H27O4PS2 [M+H]+ 391.1161, found 391.1165.
2-(4-溴苯基)-2-(二氧基硫基磷酸基)硫代)乙酸甲酯(6e): 黄色油状24.0 mg, 产率29%. 1H NMR (500 MHz, CDCl3) δ: 7.47 (d, J=8.4 Hz, 2H), 7.33 (d, J=8.4 Hz, 2H), 5.00 (d, J=13.2 Hz, 1H), 3.91~4.19 (m, 4H), 1.23~1.27 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 169.8 (d, J=6.9 Hz), 135.1 (d, J=4.7 Hz), 131.9, 130.1, 122.7, 64.3 (t, J=6.8 Hz), 53.8 (d, J=2.6 Hz), 53.2, 15.6 (d, J=8.6 Hz); HRMS (ESI-TOP) calcd for C13H18BrO4PS2Na [M+Na]+ 434.9465, found 434.9451.
2-(3-氯苯基)-2-(二氧基硫基磷基)硫代)乙酸甲酯(6f): 黄色油状22.2 mg, 产率30%. 1H NMR (500 MHz, CDCl3) δ: 7.45 (s, 1H), 7.33~7.35 (m, 1H), 7.36~7.30 (m, 2H), 5.00 (d, J=13.3 Hz, 1H), 3.89~4.19 (m, 4H), 3.74 (s, 3H), 1.23~1.28 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 169.7 (d, J=6.9 Hz), 138.0 (d, J=4.5 Hz), 134.6, 130.0, 128.7, 128.5, 126.6, 64.3 (t, J=5.4 Hz), 53.8 (d, J=2.7 Hz), 53.3, 15.6 (dd, J=8.6, 4.2 Hz); HRMS (ESI-TOP) calcd for C13H18ClO4PS2Na [M+Na]+ 390.9970, found 390.9967.
2-(二乙氧基硫基磷基)硫代)-2-(3-氟苯基)乙酸甲酯(6h): 黄色油状19.0 mg, 产率27%. 1H NMR (500 MHz, CDCl3) δ: 7.29~7.33 (m, 1H), 7.18~7.24 (m, 2H), 7.00~7.04 (m, 1H), 5.02 (d, J=13.2 Hz, 1H), 3.91~4.19 (m, 4H), 3.75 (s, 3H), 1.23~1.27 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 169.7 (d, J=6.8 Hz), 162.7 (d, J=245.9 Hz), 138.3 (dd, J=7.5, 4.7 Hz), 130.3 (d, J=8.2 Hz), 124.2 (d, J=2.9 Hz), 115.7 (d, J=8.4 Hz), 115.5 (d, J=10.6 Hz), 64.3 (t, J=5.7 Hz), 53.9 (d, J=4.4 Hz), 53.2, 15.6 (dd, J=8.6, 2.6 Hz); HRMS (ESI-TOP) calcd for C13H18FO4PS2Na [M+Na]+ 375.0266, found 375.0259.
2-(二氧基磷硫代)硫代)-2-(4-硝基苯基)乙酸乙酯 (6i): 黄色油状23.6 mg, 产率30%. 1H NMR (500 MHz, CDCl3) δ: 8.21 (d, J=8.6 Hz, 2H), 7.66 (d, J=8.7 Hz, 2H), 5.11 (d, J=13.7 Hz, 1H), 3.91~4.27 (m, 6H), 1.23~1.27 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 168.6 (d, J=7.7 Hz), 147.7, 143.5 (d, J=4.0 Hz), 129.5, 123.8, 64.5 (dd, J=9.2, 5.8 Hz), 62.7, 54.0 (d, J=2.4 Hz), 15.6 (d, J=8.4 Hz), 13.9; HRMS (ESI-TOP) calcd for C14H21N- O6PS2 [M+H]+ 394.0548, found 394.0547.
4-(1-(二乙氧基硫基磷酰基)硫代)-2-甲氧基-2-氧代乙基)苯甲酸甲酯(6j): 黄色油状21.2 mg, 产率27%. 1H NMR (500 MHz, CDCl3) δ: 8.02 (d, J=8.4 Hz, 2H), 7.53 (d, J=8.4 Hz, 2H), 5.08 (d, J=13.3 Hz, 1H), 3.97~4.18 (m, 4H), 3.91 (s, 3H), 3.74 (s, 3H), 1.21~1.27 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 169.6 (d, J=6.9 Hz), 166.4, 141.0 (d, J=4.5 Hz), 130.2, 130.0, 128.5, 64.3 (d, J=5.8 Hz), 54.4 (d, J=2.8 Hz), 53.3, 52.2, 15.6 (dd, J=8.6, 1.3 Hz); HRMS (ESI-TOP) calcd for C15H21- O6PS2Na [M+Na]+ 415.0415, found 415.0416.
2-(二氧基磷硫代)硫代)-2-苯乙酸异戊基(6k): 黄色油状23.4 mg, 产率30%. 1H NMR (500 MHz, CDCl3) δ: 7.44~7.46 (m, 2H), 7.30~7.35 (m, 3H), 5.01 (d, J=13.2 Hz, 1H), 3.83~4.16 (m, 6H), 1.86~1.94 (m, 1H), 1.26 (t, J=7.1 Hz, 3H), 1.19 (d, J=7.1 Hz, 3H), 0.86 (d, J=6.8 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 169.6 (d, J=6.8 Hz), 136.3 (d, J=4.5 Hz), 128.7, 128.5, 128.4, 72.1, 64.1 (dd, J=5.1, 0.9 Hz), 54.7 (d, J=2.8 Hz), 27.6, 18.8, 15.6 (dd, J=12.3, 8.6 Hz); HRMS (ESI-TOP) calcd for C17H27O4PS2Na [M+Na]+ 413.0986, found 413.0981.
正戊基2-((二乙氧基磷硫酰基)硫代)-2-苯乙酸酯 (6l): 黄色油状22.7 mg, 产率29%. 1H NMR (500 MHz, CDCl3) δ: 7.44~7.46 (m, 2H), 7.28~7.35 (m, 3H), 5.01 (d, J=13.2 Hz, 1H), 4.06~4.15 (m, 4H), 3.08~4.03 (m, 1H), 3.84~3.90 (m, 1H), 1.57~1.62 (m, 3H), 1.19~1.29 (m, 10H), 0.85 (d, J=6.9 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 169.7 (d, J=6.7 Hz), 136.2 (d, J=4.6 Hz), 128.7, 128.4, 128.4, 66.3, 64.1 (dd, J=5.3, 1.9 Hz), 54.7 (d, J=2.8 Hz), 28.0, 27.8, 22.1, 15.6 (dd, J=10.0, 8.7 Hz), 13.8; HRMS (ESI-TOP) calcd for C17H27O4PS2Na [M+Na]+ 413.0986, found 413.0976.
苄基2-((二乙氧基磷酸硫代)硫代)-2-苯乙酸酯(6m): 黄色油状21.4 mg, 产率26%. 1H NMR (500 MHz, CDCl3) δ: 7.42~7.44 (m, 2H), 7.24~7.33 (m, 8H), 5.04~5.19 (m, 3H), 4.02~4.14 (m, 2H), 3.81~3.99 (m, 2H), 1.16~1.22 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 169.5 (d, J=6.4 Hz), 135.9 (d, J=4.9 Hz), 135.1, 128.8, 128.6, 128.5, 128.4, 128.4, 128.1, 67.7, 64.2 (d, J=5.5 Hz), 54.5 (d, J=2.8 Hz), 15.6 (dd, J=8.4, 7.0 Hz); HRMS (ESI-TOP) calcd for C19H23O4PS2Na [M+Na]+ 433.0673, found 433.0667.
苯乙基2-((二乙氧基磷硫代)硫代)-2-苯乙酸酯(6n): 黄色油状23.7 mg, 产率28%. 1H NMR (500 MHz, CDCl3) δ: 7.38~7.40 (m, 2H), 7.30~7.32 (m, 3H), 7.19~7.24 (m, 3H), 7.08~7.09 (m, 2H), 4.98 (d, J=13.1 Hz, 1H), 4.28~4.37 (m, 2H), 4.02~4.16 (m, 2H), 3.81~3.99 (m, 2H), 2.86~2.92 (m, 2H), 1.17~1.24 (m, 6H); 13C NMR (125 MHz, CDCl3) δ: 169.5 (d, J=6.7 Hz), 137.3, 136.1 (d, J=4.6 Hz), 128.9, 128.8, 128.5, 128.5, 128.4, 126.6, 66.6, 64.2 (dd, J=5.3, 2.7 Hz), 54.6 (d, J=2.7 Hz), 34.8, 15.6 (d, J=17.5 Hz), 15.6; HRMS (ESI- TOP) calcd for C20H25O4PS2Na [M+Na]+ 447.0830, found 447.0822.
2-(二异丙氧基硫基磷基)硫代)-2-苯乙酸甲酯(6o): 黄色油状18.9 mg, 产率26%. 1H NMR (500 MHz, CDCl3) δ: 7.44~7.46 (m, 2H), 7.29~7.35 (m, 3H), 5.11 (d, J=13.8 Hz, 1H), 4.77~4.84 (m, 1H), 4.62~4.69 (m, 1H), 3.72 (s, 3H), 1.33 (d, J=6.2 Hz, 3H), 1.22 (dd, J=6.2, 1.7 Hz, 6H), 1.18 (d, J=6.2 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 170.4 (d, J=6.9 Hz), 136.2, 136.1, 128.8, 128.4, 74.0 (dd, J=14.5, 6.7 Hz), 55.0 (d, J=2.4 Hz), 53.0, 23.7 (d, J=4.7 Hz), 23.5 (d, J=4.4 Hz), 23.2 (dd, J=5.7, 1.7 Hz); HRMS (ESI-TOP) calcd for C15H23O4P- S2Na [M+Na]+ 385.0673, found 385.0658.
2-(二丁氧基硫基磷酰基)硫代)-2-苯乙酸甲酯(6p): 黄色油状23.4 mg, 产率30%. 1H NMR (500 MHz, CDCl3) δ: 7.43~7.45 (m, 2H), 7.29~7.35 (m, 3H), 5.02 (d, J=13.2 Hz, 1H), 4.05~4.11 (m, 1H), 3.97~4.03 (m, 1H), 3.81~3.91 (m, 2H), 3.73 (s, 3H), 1.53~1.58 (m, 4H), 1.29~1.36 (m, 4H), 0.89 (dt, J=7.4, 1.4 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.2 (d, J=6.7 Hz), 136.1 (d, J=4.7 Hz), 128.8, 128.5, 128.4, 67.9 (t, J=7.0 Hz), 54.5 (d, J=2.6 Hz), 53.1, 31.8 (dd, J=8.4, 2.7 Hz), 18.7 (d, J=1.0 Hz), 13.5; HRMS (ESI-TOP) calcd for C17H27O4PS2Na [M+Na]+ 413.0986, found 413.0976.
2-((双(环己氧基)磷酸硫代)硫代)-2-苯乙酸甲酯(6q): 黄色油状25.7 mg, 产率29%. 1H NMR (500 MHz, CDCl3) δ: 7.45~7.47 (m, 2H), 7.27~7.34 (m, 3H), 5.12 (d, J=13.9 Hz, 1H), 4.50~4.57 (m, 1H), 4.32~4.39 (m, 1H), 3.72 (s, 3H), 1.80~1.56 (m, 6H), 1.51~1.20 (m, 14H); 13C NMR (125 MHz, CDCl3) δ: 170.4 (d, J=7.4 Hz), 136.3 (d, J=7.4 Hz), 128.7, 128.4, 128.4, 78.6 (t, J=7.7 Hz), 55.1 (d, J=2.2 Hz), 53.0, 33.3 (d, J=4.0 Hz), 33.2 (d, J=3.8 Hz), 32.9 (dd, J=4.5, 1.5 Hz), 25.1 (d, J=4.7 Hz), 23.5; HRMS (ESI-TOP) calcd for C21H31O4PS2Na [M+Na]+ 465.1299, found 465.1289.
2-((双(己氧基)磷酸硫代)硫代)-2-苯乙酸甲酯(6r): 黄色油状25.9 mg, 产率29%. 1H NMR (500 MHz, CDCl3) δ: 7.43~7.45 (m, 2H), 7.30~7.35 (m, 3H), 5.02 (d, J=13.2 Hz, 1H), 3.96~4.10 (m, 2H), 3.79~3.90 (m, 2H), 3.72 (s, 3H), 1.54~1.60 (m, 4H), 1.25~1.31 (m, 12H), 0.88 (t, J=6.6 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.2 (d, J=6.7 Hz), 136.1 (d, J=4.7 Hz), 128.8, 128.5, 128.4, 64.2 (t, J=6.4 Hz), 54.5 (d, J=2.6 Hz), 53.1, 31.2, 29.7 (dd, J=8.4, 2.4 Hz), 25.1, 22.4, 13.9; HRMS (ESI-TOP) calcd for C21H35O4PS2Na [M+Na]+ 469.1612, found 496.1606.
2-((双(庚氧基)磷酸硫代)硫代)-2-苯乙酸甲酯(6s): 黄色油状29.5 mg, 产率31%. 1H NMR (500 MHz, CDCl3) δ: 7.43~7.45 (m, 2H), 7.30~7.35 (m, 3H), 5.02 (d, J=13.2 Hz, 1H), 3.92~4.16 (m, 2H), 3.81~3.91 (m, 2H), 3.73 (s, 3H), 1.54~1.62 (m, 4H), 1.26~1.31 (m, 16H), 0.88 (t, J=6.9 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ: 170.2 (d, J=6.7 Hz), 136.1 (d, J=4.7 Hz), 128.8, 128.5, 128.4, 68.2 (d, J=6.9 Hz), 54.5 (d, J=2.6 Hz), 53.0, 31.6, 29.8 (dd, J=8.3, 2.5 Hz), 28.8, 25.4, 22.5, 14.0; HRMS (ESI-TOP) calcd for C23H39O4PS2Na [M+Na]+ 497.1925, found 497.1920.