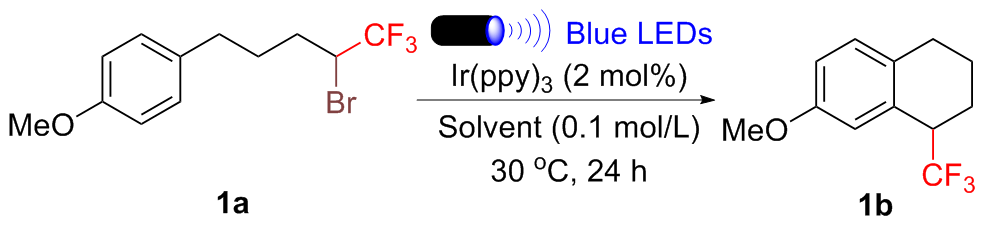
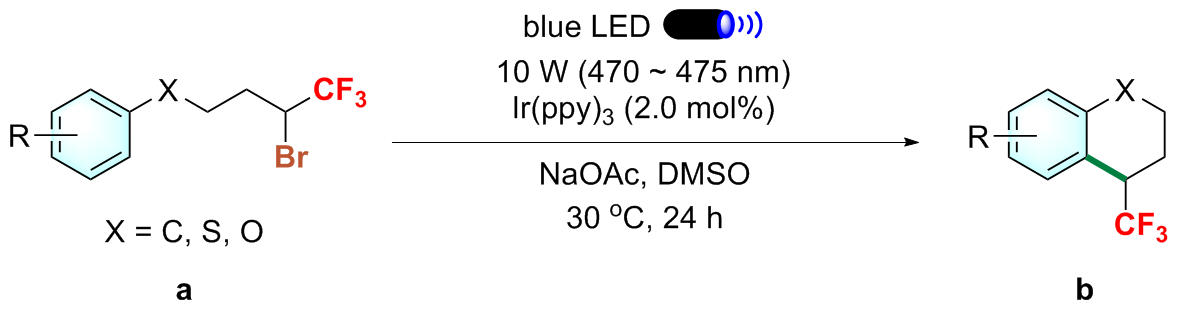
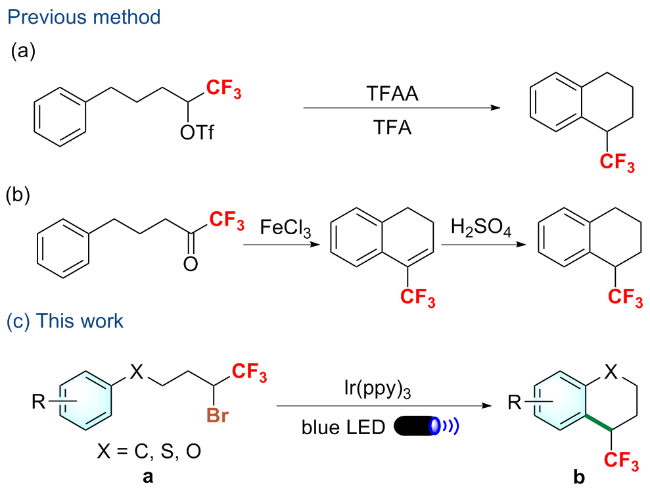
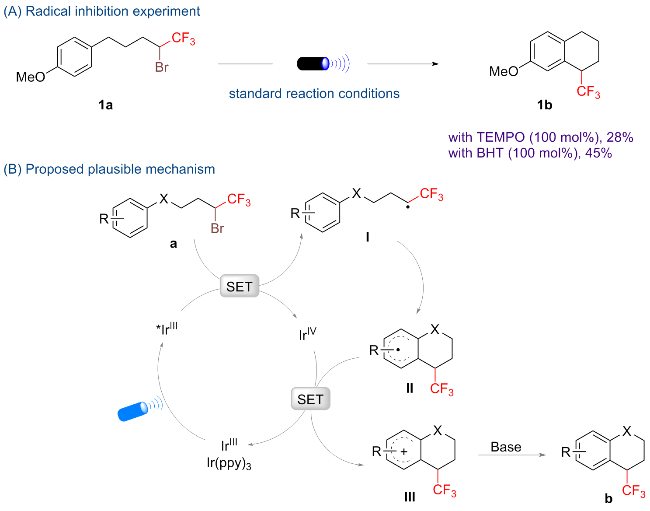
向装有磁力搅拌子的25 mL石英管中依次加入Ir(ppy)₃ (2 mol%)和NaOAc (0.4 mmol, 2.0 equiv.), 进行五次氩气抽换操作, 以排除管内空气. 随后, 向该体系中加入2.0 mL超干DMSO及化合物a (0.2 mmol, 1.0 equiv.). 将石英管密封后置于10 W的蓝色LED灯(波长范围为470~475 nm)下进行照射, 并在30 ℃的温度下搅拌24 h. 反应结束后, 以氟化苯作为内标物, 通过19F NMR测定粗产率. 用乙酸乙酯和水萃取三次, 并用饱和氯化钠溶液洗涤有机相, 无水硫酸钠干燥. 通过减压浓缩得到粗品, 以石油醚(PE)/乙酸乙酯(EA)为洗脱剂, 经硅胶柱层析分离纯化得到目标产物b.
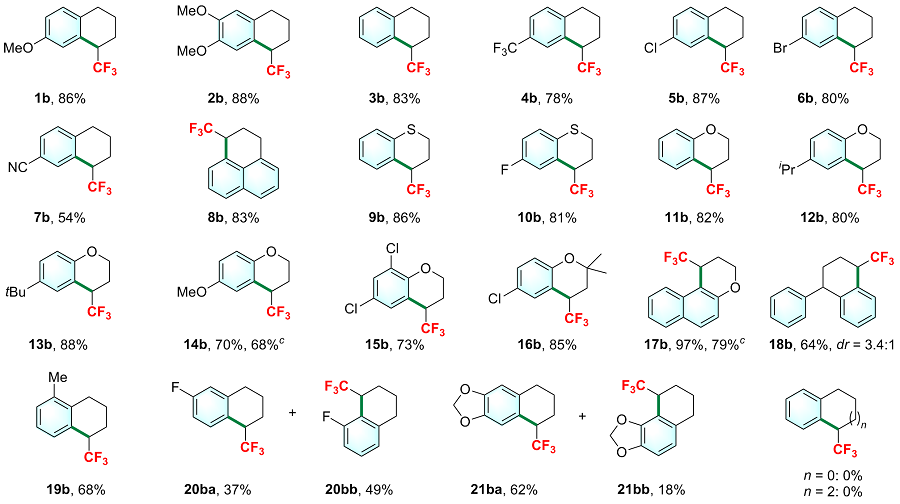
7-甲氧基-1-三氟甲基-1,2,3,4-四氢萘(1b): 产率86%, 无色液体(39.6 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.06 (d, J=8.4 Hz, 1H), 6.89 (s, 1H), 6.81 (dd, J=8.4, 2.8 Hz, 1H), 3.79 (s, 3H), 3.54~3.44 (m, 1H), 2.81~2.66 (m, 2H), 2.15~2.08 (m, 1H), 2.02~1.91 (m, 2H), 1.77~1.69 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 157.3, 130.7, 130.3, 130.2 (q, J=1.8 Hz), 127.5 (q, J=282.4 Hz), 114.9 (q, J=1.9 Hz), 113.9, 55.3, 41.8 (q, J=25.8 Hz), 28.2, 23.0 (q, J=2.5 Hz), 19.6 (d, J=0.8 Hz); 19F NMR (376 MHz, CDCl3) δ: -67.58 (d, J=9.8 Hz, 3F); HRMS (FI) calcd for C12H13F3O 230.0913, found 230.0916.
6,7-二甲氧基-1-三氟甲基-1,2,3,4-四氢萘(2b): 产率88%, 无色液体(45.8 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 6.82 (s, 1H), 6.61 (s, 1H), 3.86 (s, 3H), 3.85 (s, 3H), 3.49~3.39 (m, 1H), 2.80~2.65 (m, 2H), 2.15~2.09 (m, 1H), 2.01~1.89 (m, 2H), 1.78~1.70 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 148.4, 146.9, 131.0, 127.6 (q, J=282.4 Hz), 120.8 (q, J=2.0 Hz), 112.7 (q, J=1.9 Hz), 111.6, 55.9, 55.7, 41.2 (q, J=25.8 Hz), 28.6, 23.0 (q, J=2.5 Hz), 19.4; 19F NMR (376 MHz, CDCl3) δ: -67.79 (d, J=9.8 Hz, 3F); HRMS (EI) calcd for C13H15F3O2 260.1019, found 260.1021.
1-三氟甲基-1,2,3,4-四氢萘(
3b)
[10]: 产率83%, 无色液体(33.2 mg, 洗脱液: PE).
1H NMR (400 MHz, CDCl
3)
δ: 7.35 (d,
J=7.6 Hz, 1H), 7.25~7.13 (m, 3H), 3.56~3.48 (m, 1H), 2.88~2.73 (m, 2H), 2.20~2.12 (m, 1H), 2.04~1.93 (m, 2H), 1.79~1.72 (m, 1H);
13C NMR (101 MHz, CDCl
3)
δ: 138.7, 130.2 (q,
J=2.0 Hz), 129.5, 129.3 (q,
J=2.0 Hz), 127.6, 127.5 (q,
J=282.8 Hz), 125.7, 41.6 (q,
J=25.2 Hz), 29.0, 23.0 (dd,
J=5.0 Hz, 3.0 Hz), 19.4 (d,
J=1.0 Hz);
19F NMR (376 MHz, CDCl
3)
δ: -67.71 (d,
J=9.8 Hz, 3F); HRMS (FI) calcd for C
11H
11F
3 200.0807, found 200.0808.
1,7-双(三氟甲基)-1,2,3,4-四氢萘(4b): 产率78%, 无色液体(41.8 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.60 (s, 1H), 7.47 (d, J=8.0 Hz, 1H), 7.26 (d, J=8.0 Hz, 1H), 3.62~3.52 (m, 1H), 2.94~2.77 (m, 2H), 2.22~2.16 (m, 1H), 2.07~1.94 (m, 2H), 1.83~1.74 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 142.9, 130.1~130.0 (m), 130.0, 128.4, 127.2~127.1 (m), 127.1 (q, J=281.8 Hz), 124.4 (dd, J=7.0 Hz, 4.0 Hz), 124.1 (q, J=273.7 Hz), 41.6 (q, J=26.3 Hz), 29.0, 22.7 (dd, J=5.0, 2.0 Hz), 19.0 (d, J=1.0 Hz); 19F NMR (376 MHz, CDCl3) δ: -62.38~-62.48 (m, 3F), -67.74~-67.84 (m, 3F); HRMS (EI) calcd for C12H10F6 268.0681, found 268.0685.
7-氯-1-三氟甲基-1,2,3,4-四氢萘(5b): 产率87%, 无色液体(40.8 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.34 (s, 1H), 7.20 (dd, J=8.2 Hz, 2.2 Hz, 1H), 7.07 (d, J=8.0 Hz, 1H), 3.54~3.44 (m, 1H), 2.84~2.68 (m, 2H), 2.19~2.08 (m, 1H), 2.03~1.91 (m, 2H), 1.78~1.70 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 137.1, 131.2, 131.0~130.9 (m), 130.7, 130.0 (q, J=2.0 Hz), 127.9, 127.1 (q, J=282.8 Hz), 41.5 (q, J=26.3 Hz), 28.5, 22.7 (dd, J=5.0 Hz, 2.0 Hz), 19.2 (d, J=1.0 Hz); 19F NMR (376 MHz, CDCl3) δ: -62.71 (d, J=9.4 Hz, 3F); HRMS (FI) calcd for C11H10ClF3 234.0418, found 234.0420.
7-溴-1-三氟甲基-1,2,3,4-四氢萘(6b): 产率80%, 无色液体(44.7 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.49 (s, 1H), 7.34 (dd, J=8.2, 2.2 Hz, 1H), 7.02 (d, J=8.4 Hz, 1H), 3.54~3.44 (m, 1H), 2.82~2.66 (m, 2H), 2.17~2.08 (m, 1H), 2.02~1.90 (m, 2H), 1.78~1.69 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 137.7, 132.9 (q, J=2.0 Hz), 131.4~131.3 (m), 131.0, 130.8, 127.1 (q, J=281.8 Hz), 119.2, 41.4 (q, J=26.3 Hz), 28.5, 22.6 (dd, J=5.0, 2.0 Hz), 19.1; 19F NMR (376 MHz, CDCl3) δ: -67.69 (d, J=10.2 Hz, 3F); HRMS (FI) calcd for C11H10BrF3 277.9912, found 277.9910.
8-(三氟甲基)-5,6,7,8-四氢萘-2-甲腈(7b): 产率54%, 黄色固体(24.3 mg, 洗脱液: PE/EA, V∶V=20∶1). m.p. 65 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.65 (s, 1H), 7.50 (dd, J=8.0, 1.6 Hz, 1H), 7.25 (d, J=8.4 Hz, 1H), 3.59~3.48 (m, 1H), 2.95~2.76 (m, 2H), 2.22~2.13 (m, 1H), 2.06~1.93 (m, 2H), 1.84~1.72 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 144.6, 134.0 (q, J=2.0 Hz), 130.9~130.8 (m), 130.8~130.7 (m), 130.4 (d, J=7.1, 2.0 Hz), 126.9 (q, J=282.2 Hz), 118.7, 109.7, 41.3 (qd, J=26.7, 5.6 Hz), 29.2, 22.4 (q, J=2.5 Hz), 18.7; 19F NMR (376 MHz, CDCl3) δ: -67.79 (d, J=9.8 Hz, 3F); HRMS (FI) calcd for C12H10F3N 225.0760, found 225.0766.
1-(三氟甲基)-2,3-二氢-1H-苯丙烯(8b): 产率83%, 无色液体(39.2 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.84 (dd, J=6.8, 2.8 Hz, 1H), 7.74 (d, J=8.0 Hz, 1H), 7.49~7.41 (m, 3H), 7.32 (d, J=7.2 Hz, 1H), 3.88~3.79 (m, 1H), 3.39~3.30 (m, 1H), 3.08 (d, J=17.2 Hz, 1H), 2.56~2.49 (m, 1H), 2.22~2.12 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 134.1, 133.7, 129.7, 128.6, 127.6 (q, J=2.0 Hz), 127.3 (q, J=281.8 Hz), 127.0 (d, J=1.0 Hz), 126.3, 125.6, 125.1, 125.0, 43.1 (q, J=26.3 Hz), 26.3 (d, J=1.0 Hz), 21.9 (dd, J=5.0, 2.0 Hz); 19F NMR (376 MHz, CDCl3) δ: -67.69 (d, J=10.2 Hz, 3F); HRMS (EI) calcd for C14H11F3 236.0807, found 236.0813.
4-(三氟甲基)二氢苯并噻喃(9b): 产率86%, 无色液体(37.5 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.23 (d, J=8.0 Hz, 1H), 7.19~7.14 (m, 2H), 7.07~7.02 (m, 1H), 3.60~3.52 (m, 1H), 3.28 (td, J=13.2, 3.2 Hz, 1H), 2.95~2.87 (m, 1H), 2.61 (dq, J=14.4, 3.6 Hz, 1H), 2.17~2.04 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 134.4, 132.0 (d, J=1.0 Hz), 128.5, 126.8, 126.7 (q, J=281.8 Hz), 125.3 (d, J=2.0 Hz), 123.9, 41.4 (q, J=26.3 Hz), 22.6 (d, J=1.0 Hz), 21.9 (dd, J=5.0, 2.0 Hz); 19F NMR (376 MHz, CDCl3) δ: -67.61 (d, J=9.4 Hz, 3F); HRMS (EI) calcd for C10H9F3S 218.0372, found 218.0374.
6-氟-4-(三氟甲基)二氢苯并噻喃(10b): 产率81%, 无色液体(38.3 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.12 (dd, J=8.8, 5.6 Hz, 1H), 7.05~6.90 (m, 2H), 3.58~3.48 (m, 1H), 3.24 (td, J=12.8, 3.6 Hz, 1H), 2.95~2.88 (m, 1H), 2.57 (dq, J=14.8, 4.0 Hz, 1H), 2.17~2.05 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 159.5 (d, J=244.4 Hz), 129.5 (d, J=4.0 Hz), 128.1 (d, J=7.1 Hz), 127.0 (dd, J=7.1, 1.0 Hz), 126.5 (q, J=282.8 Hz), 118.4 (dd, J=23.2, 2.0 Hz), 116.2 (d, J=22.2 Hz), 41.6 (qd, J=27.3, 2.0 Hz), 22.6 (d, J=1.0 Hz), 21.9 (q, J=2.0 Hz); 19F NMR (376 MHz, CDCl3) δ: -67.58 (d, J=9.8 Hz, 3F), -118.71~-118.83 (m, 1F); HRMS (EI) calcd for C10H8F4S 236.0277, found 236.0279.
4-(三氟甲基)色烷(11b): 产率82%, 无色液体(33.2 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.30 (d, J=7.2 Hz, 1H), 7.26~7.20 (m, 1H), 6.92 (td, J=7.6, 1.2 Hz, 1H), 6.88 (d, J=8.4 Hz, 1H), 4.27~4.21 (m, 2H), 3.57~3.47 (m, 1H), 2.31~2.14 (m, 2H); 13C NMR (101 MHz, CDCl3) δ: 155.4, 130.7 (d, J=2.0 Hz), 129.6, 126.8 (q, J=280.8 Hz), 120.5, 117.5, 114.5~114.4 (m), 62,5 (d, J=1.0 Hz), 37.7 (q, J=27.3 Hz), 22.0 (q, J=2.0 Hz); 19F NMR (376 MHz, CDCl3) δ: -68.67 (d, J=9.8 Hz, 3F); HRMS (EI) calcd for C10H9F3O 202.0600, found 202.0594.
6-异丙基-4-(三氟甲基)色烷(12b): 产率80%, 无色液体(39.1 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.15~7.13 (m, 1H), 7.11 (dd, J=8.0, 2.0 Hz, 1H), 6.82 (d, J=8.4 Hz, 1H), 4.24~4.19 (m, 2H), 3.54~3.45 (m, 1H), 2.90~2.80 (m, 1H), 2.30~2.13 (m, 2H), 1.24 (d, J=2.0 Hz, 3H), 1.22 (d, J=2.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 153.3, 140.8, 128.4 (d, J=2.0 Hz), 127.6, 126.8 (q, J=281.8 Hz), 117.2, 114.0~113.9 (m), 62.5 (d, J=1.0 Hz), 37.7 (q, J=27.3 Hz), 33.2, 24.2, 24.0, 22.1 (q, J=2.0 Hz); 19F NMR (376 MHz, CDCl3) δ: -69.74 (d, J=9.4 Hz, 3F); HRMS (FI) calcd for C13H15F3O 244.1070, found 244.1068.
6-(叔丁基)-4-(三氟甲基)色烷(13b): 产率88%, 无色液体(45.5 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.31~7.25 (m, 2H), 6.83 (d, J=8.4 Hz, 1H), 4.25~4.20 (m, 2H), 3.56~3.45 (m, 1H), 2.30~2.13 (m, 2H), 1.30 (s, 9H); 13C NMR (101 MHz, CDCl3) δ: 153.0, 143.1, 127.4 (q, J=1.0 Hz), 126.8 (q, J=281.8 Hz), 126.7, 116.8, 113.6~113.5 (m), 62.4, 37.7 (q, J=27.3 Hz), 34.0, 31.4, 22.1 (q, J=2.0 Hz); 19F NMR (376 MHz, CDCl3) δ: -69.70~-69.82 (m, 3F); HRMS (FI) calcd for C14H17- F3O 258.1226, found 258.1224.
6-甲氧基-4-(三氟甲基)色烷(14b): 产率70%, 无色液体(32.5 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 6.85~6.79 (m, 3H), 4.21~4.16 (m, 2H), 3.76 (s, 3H), 3.54~3.44 (m, 1H), 2.28~2.12 (m, 2H); 13C NMR (101 MHz, CDCl3) δ: 153.2, 149.5, 126.7 (q, J=281.8 Hz), 118.1, 116.1, 114.7 (q, J=2.0 Hz), 114.7~114.6 (m), 62.5 (d, J=1.0 Hz), 55.7, 37.9 (q, J=27.3 Hz), 22.2 (q, J=2.0 Hz); 19F NMR (376 MHz, CDCl3) δ: -69.78 (d, J=9.4 Hz, 3F); HRMS (FI) calcd for C11H11F3O2 232.0706, found 232.0705.
6,8-二氯-4-(三氟甲基)色烷(15b): 产率73%, 无色液体(39.6 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.35 (d, J=2.4 Hz, 1H), 7.22~7.20 (m, 1H), 4.43~ 4.37 (m, 1H), 4.35~4.26 (m, 1H), 3.56~3.44 (m, 1H), 2.34~2.27 (m, 1H), 2.24~2.13 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 150.0, 131.1, 128.8 (q, J=2.0 Hz), 126.2 (q, J=281.8 Hz), 124.9, 123.2, 117.0~116.9 (m), 63.4 (d, J=1.0 Hz), 37.7 (q, J=28.3 Hz), 21.5 (q, J=2.0 Hz); 19F NMR (376 MHz, CDCl3) δ: -68.44 (d, J=9.4 Hz, 3F); HRMS (EI) calcd for C10H7Cl2F3O 269.9821, found 269.9814.
6-氯-2,2-二甲基-4-(三氟甲基)色烷(16b): 产率85%, 无色液体(45.0 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.44 (s, 1H), 7.16 (dd, J=8.8, 2.4 Hz, 1H), 6.78 (d, J=8.8 Hz, 1H), 3.67~3.55 (m, 1H), 2.12~2.05 (m, 1H), 2.00~1.91 (m, 1H), 1.48 (s, 3H), 1.22 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 152.9, 129.4 (dd, J=7.5, 2.2 Hz), 127.8~127.5 (m), 127.0 (q, J=281.1 Hz), 125.3, 119.7~119.6 (m), 116.1 (d, J=1.7 Hz), 73.7, 37.4 (qd, J=28.1, 6.8 Hz), 33.3 (d, J=2.4 Hz), 29.6 (d, J=3.7 Hz), 23.3 (d, J=1.9 Hz); 19F NMR (376 MHz, CDCl3) δ: -68.10 (d, J=8.3 Hz, 3F); HRMS (FI) calcd for C12H12- ClF3O 264.0523, found 264.0520.
1-(三氟甲基)-2,3-二氢-1H-苯并[f]色烯(17b): 产率 97%, 无色液体(48.9 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.92 (d, J=8.4 Hz, 1H), 7.79 (d, J=8.4 Hz, 1H), 7.76 (d, J=9.2 Hz, 1H), 7.57~7.51 (m, 1H), 7.41~7.34 (m, 1H), 7.10 (d, J=8.8 Hz, 1H), 4.52~4.42 (m, 2H), 4.25~4.12 (m, 1H), 2.51~2.42 (m, 1H), 2.26~2.11 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 153.3, 132.9, 130.6, 129.0, 128.5, 127.1 (q, J=281.8 Hz), 126.8, 123.3, 122.2 (q, J=3.0 Hz), 119.1, 106.0~105.9 (m), 61.8 (d, J=2.0 Hz), 34.0 (q, J=27.3 Hz), 21.5 (q, J=2.0 Hz); 19F NMR (376 MHz, CDCl3) δ: -66.74~-66.90 (m, 3F); HRMS (FI) calcd for C14H11F3O 252.0757, found 252.0763.
1-苯基-4-三氟甲基-1,2,3,4-四氢萘(18b): 产率64%, 无色液体(35.4 mg, 洗脱液: PE). Major: 1H NMR (400 MHz, CDCl3) δ: 7.47 (d, J=7.2 Hz, 1H), 7.36~7.15 (m, 6H), 7.01 (d, J=7.2 Hz, 2H), 4.22 (t, J=5.6 Hz, 1H), 3.73~3.55 (m, 1H), 2.46~2.35 (m, 1H), 2.18~1.99 (m, 2H), 1.90~1.80 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 146.6, 140.7, 130.7~130.6 (m), 129.9~129.8 (m), 128.7~128.5 (m), 128.4~128.2 (m), 127.9~127.8 (m), 127.5 (q, J=282.8 Hz), 126.4~126.3 (m), 126.2~126.1 (m), 44.3 (d, J=6.1 Hz), 41.8 (dq, J=26.3, 7.1 Hz), 28.7, 19.6 (q, J=2.0 Hz); 19F NMR (376 MHz, CDCl3) δ: -67.68 (d, J=7.9 Hz, 3F); HRMS (FI) calcd for C17H15F3 276.1120, found 276.1118.
5-甲基-1-三氟甲基-1,2,3,4-四氢萘(19b): 产率68%, 无色液体(29.1 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.24 (d, J=6.8 Hz, 1H), 7.18~7.10 (m, 2H), 3.60~3.48 (m, 1H), 2.82~2.72 (m, 1H), 2.68~2.56 (m, 1H), 2.27 (s, 3H), 2.25~2.17 (m, 1H), 2.09~1.98 (m, 1H), 1.97~1.82 (m, 2H); 13C NMR (101 MHz, CDCl3) δ: 137.0, 136.8, 129.3, 129.2 (d, J=1.5 Hz), 128.2 (d, J=1.1 Hz), 127.5 (q, J=282.1 Hz), 125.2, 41.5 (q, J=25.6 Hz), 26.0, 22.5 (q, J=2.5 Hz), 19.7, 18.9; 19F NMR (376 MHz, CDCl3) δ: -67.65~-67.98 (m, 3F); HRMS (EI) calcd for C12H13F3 214.0964, found 214.0968.
6-氟-1-三氟甲基-1,2,3,4-四氢萘(20ba)和8-氟-1-三氟甲基-1,2,3,4-四氢萘(20bb)混合物: 产率86% (20ba, 37%; 20bb, 49%), 无色液体(37.6 mg, 洗脱液: PE). 1H NMR (400 MHz, CDCl3) δ: 7.32 (t, J=7.2 Hz, 1H, 20ba), 7.25~7.18 (m, 1H, 20bb), 6.99~6.82 (m, 2H, mixed), 3.93~3.81 (m, 1H, 20bb), 3.56~3.44 (m, 1H, 20ba), 2.96~2.70 (m, 2H, mixed), 2.33~2.23 (m, 1H, 20bb), 2.22~2.12 (m, 1H, 20ba), 2.12~1.66 (m, 3H, mixed); 13C NMR (101 MHz, CDCl3) δ: 162.0 (d, J=247.6 Hz, 20ba), 161.3 (d, J=249.3 Hz, 20bb), 141.3 (d, J=2.5 Hz, 20bb), 141.1 (d, J=7.1 Hz, 20ba), 131.9 (dd, J=8.2, 2.1 Hz, 20ba), 128.9 (d, J=10.1 Hz, 20bb), 127.4 (q, J=281.7 Hz, 20ba), 127.2 (q, J=282.6 Hz, 20bb), 125.0~124.9 (m, 20ba), 124.7 (d, J=3.0 Hz, 20bb), 117.5 (dd, J=16.1, 2.0 Hz, 20bb), 115.6 (d, J=20.2 Hz, 20ba), 113.0 (d, J=21.5 Hz, 20ba), 112.5 (d, J=22.6 Hz, 20bb), 41.1 (q, J=26.1 Hz, 20ba), 35.3 (qd, J=27.6, 1.3 Hz, 20bb), 29.1 (20ba), 27.8 (d, J=2.5 Hz, 20bb), 22.8 (q, J=2.2 Hz, 20ba), 21.8 (q, J=2.2 Hz, 20bb), 19.0 (20ba), 18.4 (20bb); 19F NMR (376 MHz, CDCl3) δ: -68.15~-68.45 (m, 3F, 20bb),-68.62~-68.92 (m, 3F, 20ba), -115.70~-115.95 (m, 1F, 20ba), -116.45~-116.75 (m, 1F, 20bb); HRMS (EI) calcd for C11H10F4 218.0713, found 218.0717.
5-(三氟甲基)-5,6,7,8-四氢萘并[2,3-
d][
1,
3]二噁茂(
21ba)和9-(三氟甲基)-6,7,8,9-四氢萘并[1,2-
d][
1,
3]二噁茂(
21bb)混合物: 产率80% (
21ba, 62%;
21bb, 18%), 无色液体(39.1 mg, 洗脱液: PE/EA,
V∶
V=20∶1).
1H NMR (400 MHz, CDCl
3)
δ: 6.82 (s, 1H,
21ba), 6.74 (d,
J=8.0 Hz, 1H,
21bb), 6.63 (d,
J=8.0 Hz, 1H,
21bb), 6.60 (s, 1H,
21ba), 5.95 (d,
J=41.2 Hz, 2H,
21bb), 5.92 (d,
J=6.4 Hz, 2H,
21ba), 3.74~3.63 (m, 1H,
21bb), 3.47~3.36 (m, 1H,
21ba), 2.86~2.62 (m, 2H, mixed), 2.26~2.18 (m, 1H,
20bb), 2.16~2.08 (m, 1H,
20ba), 2.05~1.80 (m, 2H, mixed), 1.78~1.66 (m, 1H, mixed);
13C NMR (101 MHz, CDCl
3)
δ: 147.1 (
21ba), 146.5 (
21bb), 145.7 (
21ba), 145.1 (
21bb), 132.40 (
21bb), 132.37 (
21ba), 127.5 (q,
J=282.2 Hz,
21ba), 127.4 (q,
J=282.9 Hz,
21bb), 121.8 (q,
J=2.0 Hz,
21ba), 121.4 (
21bb), 112.0 (q,
J=1.8 Hz,
21bb), 109.8 (q,
J=2.1 Hz,
21ba), 108.9 (
21ba), 108.1 (
21bb), 100.9, 41.6 (q,
J=25.8 Hz,
21ba), 36.4 (q,
J=27.7 Hz,
21bb), 29.1 (
21ba), 27.8 (
21bb), 22.9 (q,
J=2.6 Hz,
21ba), 21.9 (q,
J=2.3 Hz,
21bb), 19.3 (
21ba), 19.1 (
21bb);
19F NMR (376 MHz, CDCl
3)
δ: -67.54 (d,
J=9.8 Hz, 3F,
21bb), -67.93 (d,
J=9.8 Hz, 3F,
21ba); HRMS (FI) calcd for C
12H
11O
2F
3 244.0706, found 244.0707.