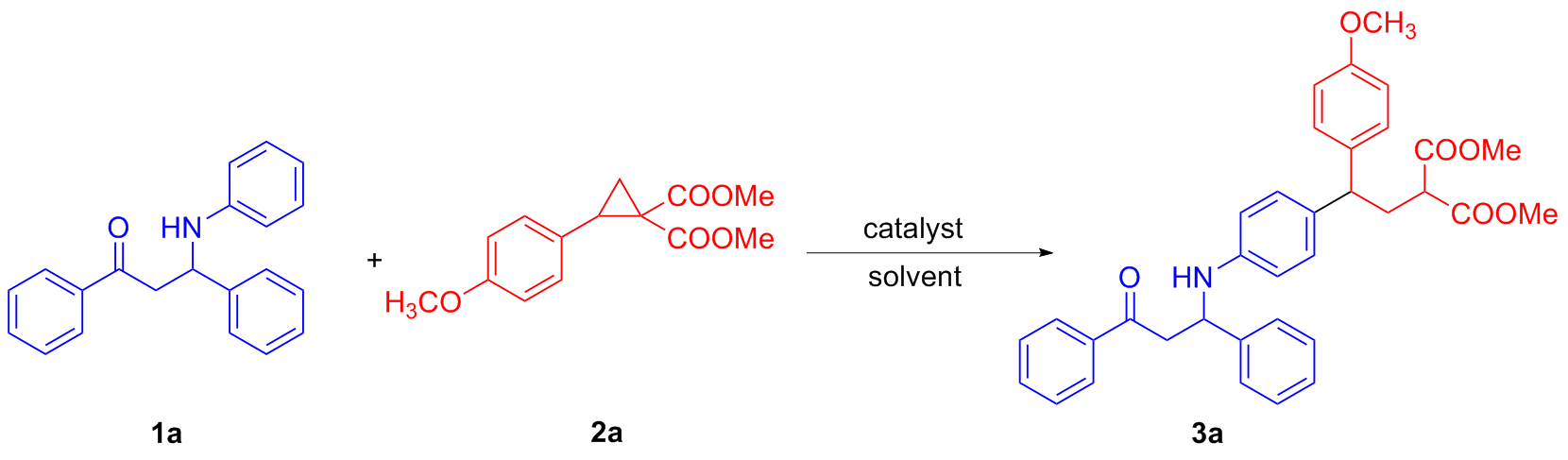
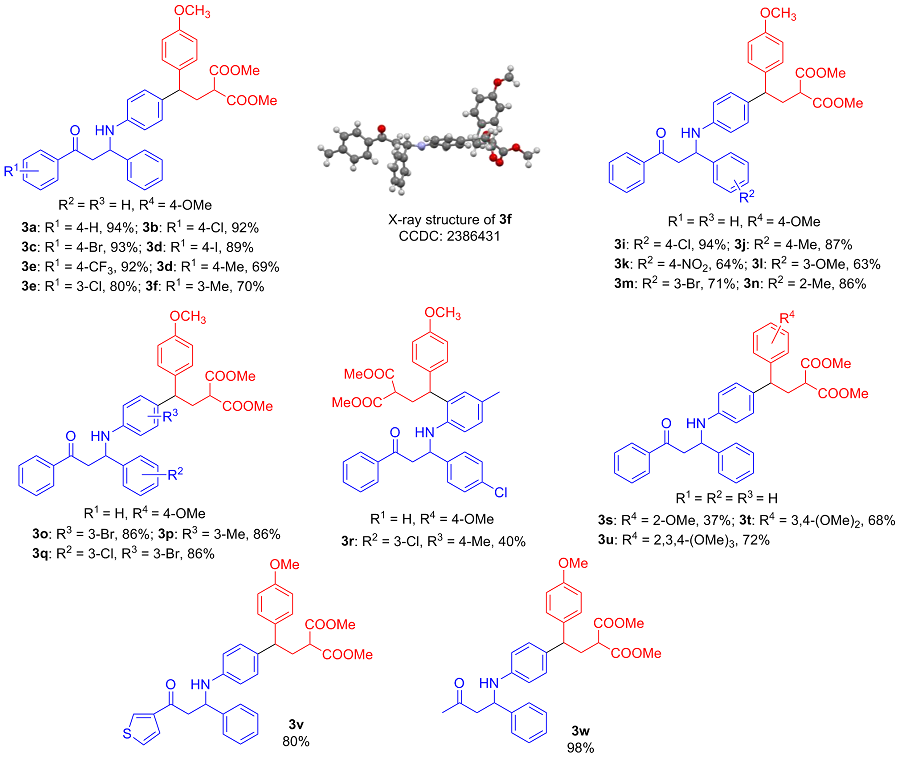
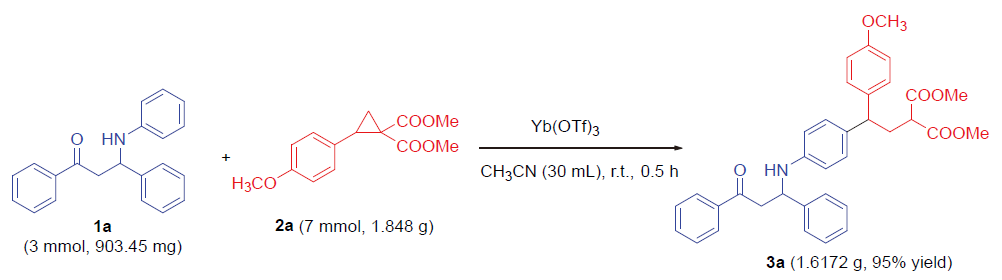
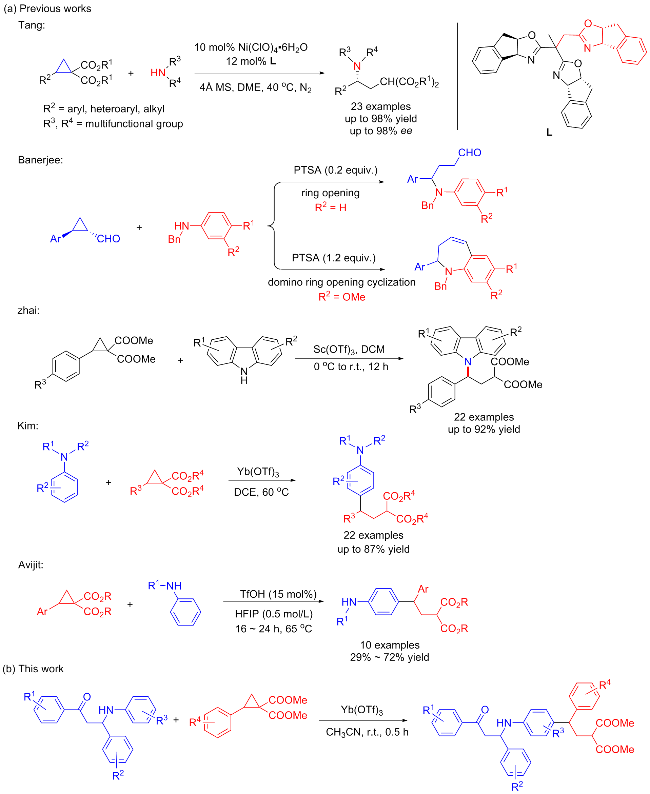
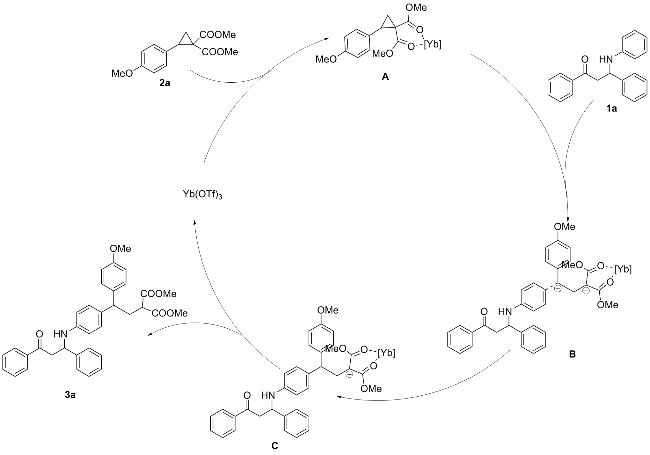
称取三氟甲磺酸镱(0.05 mmol, 31.0 mg)、全芳基β-氨基酮1a (0.1 mmol, 30.1 mg)和D-A环丙烷2a (0.25 mmol, 66.0 mg), 并将其依次加入反应管中, 再加入2 mL乙腈, 常温下反应0.5 h, 薄层色谱(TLC)检测反应结束后, 通过硅胶柱层析(石油醚/乙酸乙酯, V∶V=5∶1)分离得到无色油状的目标化合物3a. 以相同的方法合成了化合物3b~3w.
2-(2-(4-甲氧基苯基)-2-(4-((3-氧代-1,3-二苯基丙基)氨基)基)乙基)丙二酸二甲酯(3a): 无色油状物, 产率94%. 1H NMR (400 MHz, CDCl3) δ: 7.88 (d, J=7.2 Hz, 2H), 7.54 (t, J=7.2 Hz, 1H), 7.44~7.40 (m, 4H), 7.30 (t, J=7.6 Hz, 2H), 7.22 (t, J=7.6 Hz, 1H), 7.07 (d, J=8.8 Hz, 2H), 6.91 (d, J=8.8 Hz, 2H), 6.77 (d, J=8.8 Hz, 2H), 6.47 (d, J=8.8 Hz, 2H), 4.97~4.90 (m, 1H), 4.48 (s, 1H), 3.73 (s, 3H), 3.69 (d, J=7.6 Hz, 1H), 3.66 (d, J=2.0 Hz, 3H), 3.64 (s, 3H), 3.46 (dd, J=16.0, 5.2 Hz, 1H), 3.37 (dd, J=16.0, 8.0 Hz, 1H), 3.24 (t, J=7.6 Hz, 1H), 2.52 (t, J=7.6 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ: 198.2, 169.9, 158.0, 145.6, 143.1, 136.6, 136.2, 136.2, 133.4, 132.8, 128.8, 128.7, 128.4, 128.2, 127.3, 126.3, 113.9, 113.8, 55.2, 54.9, 52.5, 50.1, 47.0, 46.4, 34.9, 34.8; HRMS (ESI) calcd for C35H35NO6Br (M+H)+ 566.2537, found 566.2557.
2-(2-(4-((3-(4-氯苯基)-3-氧代-1-苯丙基)氨基)苯基)-2-(4-甲氧基苯基)乙基)丙二酸二甲酯(3b): 无色油状物, 产率92%. 1H NMR (400 MHz, CDCl3) δ: 7.80 (d, J=8.8 Hz, 2H), 7.39 (dd, J=8.0, 3.6 Hz, 4H), 7.30 (t, J=7.6 Hz, 2H), 7.22 (t, J=7.2 Hz, 1H), 7.07 (d, J=8.8 Hz, 2H), 6.91 (d, J=8.4 Hz, 2H), 6.77 (d, J=8.6 Hz, 2H), 6.47 (d, J=8.4 Hz, 2H), 4.96~4.90 (m, 1H), 4.44 (s, 1H), 3.74 (s, 3H), 3.70 (d, J=8.4 Hz, 1H), 3.66 (s, 3H), 3.64 (s, 3H), 3.45~3.32 (m, 2H), 3.24 (t, J=6.4 Hz, 1H), 2.52 (t, J=8.8 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ: 197.0, 169.9, 169.9, 158.0, 145.4, 142.9, 139.9, 136.2, 135.0, 133.0, 129.6, 129.0, 128.9, 128.7, 128.5, 127.5, 126.4, 114.0, 113.9, 55.2, 54.9, 52.5, 50.1, 47.0, 46.3, 34.9, 34.9; HRMS (ESI) calcd for C35H35NO6Cl (M+H)+ 600.2147, found 600.2145.
2-(2-(4-((3-(4-溴苯基)-3-氧代-1-苯丙基)氨基)苯基)-2-(4-甲氧基苯基)乙基)丙二酸二甲酯(3c): 无色油状物, 产率93%. 1H NMR (400 MHz, CDCl3) δ: 7.89 (d, J=7.2 Hz, 2H), 7.56 (d, J=11.6 Hz, 2H), 7.44 (t, J=7.6 Hz, 2H), 7.36 (d, J=7.6 Hz, 2H), 7.18 (t, J=7.6 Hz, 1H), 7.08 (d, J=8.8 Hz, 2H), 6.92 (d, J=8.4 Hz, 2H), 6.78 (d, J=8.8 Hz, 2H), 6.45 (d, J=8.4 Hz, 2H), 4.93~4.84 (m, 1H), 4.47 (s, 1H), 3.75 (s, 3H), 3.71 (d, J=8.0 Hz, 1H), 3.67 (d, J=2.0 Hz, 3H), 3.65 (s, 3H), 3.44 (dd, J=16.4, 5.2 Hz, 1H), 3.36 (dd, J=16.4, 7.6 Hz, 1H), 3.25 (t, J=7.2 Hz, 1H), 2.52 (t, J=7.2 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ: 197.7, 169.9, 169.9, 158.0, 145.8, 145.2, 136.5, 136.1, 133.6, 133.2, 130.6, 130.5, 129.4, 128.8, 128.5, 128.2, 125.1, 123.0, 113.9, 55.2, 54.6, 52.5, 50.1, 47.0, 46.2, 34.87; HRMS (ESI) calcd for C35H35NO6Br (M+H)+ 644.1642, found 644.1643.
2-(2-(4-((3-(4-碘苯基)-3-氧代-1-苯丙基)氨基)苯基)-2-(4-甲氧基苯基)乙基)丙二酸二甲酯(3d): 无色油状物, 产率89%. 1H NMR (400 MHz, CDCl3) δ: 7.79 (s, 2H), 7.56 (d, J=8.4 Hz, 2H), 7.39 (d, J=7.2 Hz, 2H), 7.30 (t, J=7.6 Hz, 2H), 7.22 (t, J=7.2 Hz, 1H), 7.07 (d, J=8.4 Hz, 2H), 6.91 (d, J=8.4 Hz, 2H), 6.76 (s, 2H), 6.45 (s, 2H), 4.95~4.89 (m, 1H), 4.42 (s, 1H), 3.74 (s, 3H), 3.70 (dd, J=5.2, 2.0 Hz, 1H), 3.66 (d, J=2.0 Hz, 3H), 3.65 (d, J=1.2 Hz, 3H), 3.42~3.30 (m, 2H), 3.24 (td, J=7.2, 2.0 Hz, 1H), 2.52 (t, J=7.6 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ: 197.6, 169.8, 169.9, 158.0, 145.4, 142.9, 138.0, 136.2, 135.9, 133.0, 129.6, 128.9, 128.7, 128.5, 127.5, 126.4, 114.0, 113.9, 101.5, 55.2, 54.9, 52.5, 50.1, 47.0, 46.2, 34.9, 34.9; HRMS (ESI) calcd for C35H35NO6I (M+H)+ 692.1504, found 692.1506.
2-(2-(4-甲氧基苯基)-2-(4-((3-氧代-1-苯基-3-(4-(三氟甲基)苯基)丙基)氨基)苯基)乙基)丙二酸二甲酯(3e): 无色油状物, 产率92%. 1H NMR (400 MHz, CDCl3) δ: 7.96 (d, J=8.4 Hz, 2H), 7.68 (d, J=8.4 Hz, 2H), 7.40 (d, J=7.2 Hz, 2H), 7.31 (t, J=7.6 Hz, 2H), 7.25~7.22 (m, 1H), 7.08 (d, J=8.8 Hz, 2H), 6.93 (d, J=8.4 Hz, 2H), 6.78 (d, J=8.8 Hz, 2H), 6.48 (d, J=8.4 Hz, 2H), 4.96 (t, J=6.4 Hz, 1H), 4.39 (s, 1H), 3.74 (s, 3H), 3.71 (d, J=8.8 Hz, 1H), 3.67 (d, J=2.0 Hz, 3H), 3.65 (s, 3H), 3.50~3.39 (m, 2H), 3.25 (td, J=7.2, 1.6 Hz, 1H), 2.52 (t, J=7.6 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ: 197.4, 169.8, 169.9, 158.1, 145.3, 142.7, 139.3, 136.1, 134.6 (d, J=32.3 Hz), 133.1, 128.9, 128.7, 128.6, 128.5, 128.5, 127.6, 126.4, 125.8 (q, J=3.0 Hz), 124.8, 122.2, 114.0, 113.9, 55.2, 54.7, 52.5, 50.2, 47.4, 46.5, 34.9, 34.9; HRMS (ESI) calcd for C36H35F3NO6 (M+H)+ 634.2411, found 634.2413.
2-(2-(4-甲氧基苯基)-2-(4-((3-氧代-1-苯基-3-(对甲苯基)丙基)氨基)苯基)乙基)丙二酸二甲酯(3f): 白色固体, 产率69%. m.p. 116.0~122.7 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.79 (d, J=8.4 Hz, 2H), 7.41 (d, J=7.2 Hz, 2H), 7.31 (t, J=7.6 Hz, 2H), 7.23 (d, J=8.4 Hz, 3H), 7.07 (d, J=8.8 Hz, 2H), 6.90 (d, J=8.4 Hz, 2H), 6.77 (d, J=8.4 Hz, 2H), 6.46 (d, J=8.4 Hz, 2H), 4.94~4.88 (m, 1H), 4.51 (s, 1H), 3.74 (s, 3H), 3.70 (t, J=8.0 Hz, 1H), 3.66 (d, J=2.4 Hz, 3H), 3.65 (d, J=1.2 Hz, 3H), 3.44 (dd, J=16.0, 4.8 Hz, 1H), 3.33 (dd, J=16.0, 8.0 Hz, 1H), 3.24 (td, J=7.2, 2.0 Hz, 1H), 2.55~2.48 (m, 2H), 2.39 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 197.9, 169.9, 158.0, 145.6, 144.3, 143.2, 136.2, 134.2, 132.8, 129.4, 128.8, 128.7, 128.4, 128.4, 127.3, 126.4, 114.0, 113.9, 55.2, 55.1, 52.5, 50.1, 47.0, 46.3, 34.9, 21.7; HRMS (ESI) calcd for C36H38NO6 (M+H)+ 580.2694, found 580.2691.
2-(2-(4-((3-(3-氯苯基)-3-氧代-1-苯丙基)氨基)苯基)-2-(4-甲氧基苯基)乙基)丙二酸二甲酯(3g): 无色油状物, 产率80%. 1H NMR (400 MHz, CDCl3) δ: 7.84 (t, J=2.0 Hz, 1H), 7.74 (d, J=7.6 Hz, 1H), 7.51 (d, J=8.0 Hz, 1H), 7.40 (d, J=7.2 Hz, 2H), 7.36 (t, J=8.0 Hz, 1H), 7.31 (t, J=7.6 Hz, 2H), 7.25~7.20 (m, 1H), 7.08 (d, J=8.8 Hz, 2H), 6.92 (d, J=8.4 Hz, 2H), 6.78 (d, J=8.4 Hz, 2H), 6.48 (d, J=8.4 Hz, 2H), 4.94 (t, J=6.4 Hz, 1H), 4.42 (s, 1H), 3.74 (s, 3H), 3.70 (d, J=6.8 Hz, 1H), 3.67 (d, J=2.0 Hz, 3H), 3.65 (d, J=0.8 Hz, 3H), 3.46~3.34 (m, 2H), 3.25 (td, J=7.2, 1.6 Hz, 1H), 2.57~2.47 (m, 2H); 13C NMR (101 MHz, CDCl3) δ: 196.9, 169.8, 169.9, 158.0, 145.4, 142.8, 138.2, 136.2, 135.1, 133.3, 133.0, 130.0, 128.9, 128.7, 128.5, 128.3, 127.5, 126.4, 126.3, 114.0, 113.9, 55.2, 54.8, 52.5, 50.1, 47.0, 46.4, 34.8; HRMS (ESI) calcd for C35H35NO6Cl (M+H)+ 600.2147, found 600.2146.
2-(2-(4-甲氧基苯基)-2-(4-((3-氧代-1-苯基-3-(间甲苯基)丙基)氨基)苯基)乙基)丙二酸二甲酯(3h): 无色油状物, 产率70%. 1H NMR (400 MHz, CDCl3) δ: 7.68 (d, J=5.6 Hz, 2H), 7.42 (d, J=7.2 Hz, 2H), 7.36 (d, J=7.8 Hz, 1H), 7.31 (t, J=8.0 Hz, 3H), 7.22 (t, J=7.2 Hz, 1H), 7.07 (d, J=8.4 Hz, 2H), 6.91 (d, J=8.4 Hz, 2H), 6.77 (d, J=8.4 Hz, 2H), 6.47 (d, J=8.4 Hz, 2H), 4.92 (dd, J=7.6, 5.2 Hz, 1H), 4.48 (s, 1H), 3.74 (s, 3H), 3.71 (d, J=8.0 Hz, 1H), 3.66 (d, J=2.0 Hz, 3H), 3.65 (d, J=1.2 Hz, 3H), 3.45 (dd, J=16.0, 5.2 Hz, 1H), 3.36 (dd, J=16.0, 7.6 Hz, 1H), 3.25 (td, J=7.2, 1.6 Hz, 1H), 2.52 (t, J=7.8 Hz, 2H), 2.37 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 198.4, 169.9, 169.9, 158.0, 145.6, 143.2, 138.5, 136.7, 136.2, 134.2, 132.8, 128.8, 128.8, 128.7, 128.5, 128.4, 127.3, 126.4, 125.4, 113.9, 113.9, 55.2, 55.0, 52.5, 50.1, 47.0, 46.5, 34.9, 21.3; HRMS (ESI) calcd for C36H38NO6 (M+H)+ 580.2694, found 580.2692.
2-(2-(4-((1-(4-氯苯基)-3-氧代-1-苯丙基)氨基)苯基)-2-(4-甲氧基苯基)乙基)丙二酸二甲酯(3i): 无色油状物, 产率94%. 1H NMR (400 MHz, CDCl3) δ: 7.88 (d, J=7.2 Hz, 2H), 7.56 (t, J=7.6 Hz, 1H), 7.44 (t, J=7.6 Hz, 2H), 7.36 (d, J=8.4 Hz, 2H), 7.29~7.25 (m, 2H), 7.07 (d, J=8.4 Hz, 2H), 6.92 (d, J=8.0 Hz, 2H), 6.78 (d, J=8.4 Hz, 2H), 6.44 (d, J=8.4 Hz, 2H), 4.91 (t, J=6.4 Hz, 1H), 4.49 (s, 1H), 3.74 (s, 3H), 3.70 (d, J=7.6 Hz, 1H), 3.67 (d, J=2.0 Hz, 3H), 3.66 (s, 3H), 3.47~3.33 (m, 2H), 3.25 (t, J=6.4 Hz, 1H), 2.52 (t, J=7.6 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ: 197.9, 169.9, 169.8, 158.0, 145.3, 141.7, 136.5, 136.1, 133.6, 133.2, 133.0, 129.0, 128.8, 128.7, 128.5, 128.2, 127.8, 114.0, 113.8, 55.2, 54.3, 52.5, 50.1, 47.0, 46.2, 34.9, 34.8; HRMS (ESI) calcd for C35H35NO6Cl (M+H)+ 600.2147, found 600.2144.
2-(2-(4-甲氧基苯基)-2-(4-((3-氧代-3-苯基-1-(对甲苯基)丙基)氨基)苯基)乙基)丙二酸二甲酯(3j): 无色油状物, 产率87%. 1H NMR (400 MHz, CDCl3) δ: 7.89 (d, J=7.2 Hz, 2H), 7.55 (t, J=7.2 Hz, 1H), 7.43 (t, J=7.6 Hz, 2H), 7.30 (d, J=8.0 Hz, 2H), 7.12 (d, J=7.6 Hz, 2H), 7.07 (d, J=8.8 Hz, 2H), 6.91 (d, J=8.4 Hz, 2H), 6.78 (d, J=8.8 Hz, 2H), 6.47 (d, J=8.4 Hz, 2H), 4.90 (dd, J=7.6, 5.2 Hz, 1H), 4.45 (s, 1H), 3.74 (s, 3H), 3.72~3.69 (m, 1H), 3.67 (d, J=1.6 Hz, 3H), 3.65 (s, 3H), 3.46 (dd, J=16.0, 5.2 Hz, 1H), 3.35 (dd, J=16.0, 7.6 Hz, 1H), 3.28~3.21 (m, 1H), 2.52 (t, J=7.6 Hz, 2H), 2.30 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 198.3, 169.9, 158.0, 145.6, 140.1, 134.0, 136.7, 136.3, 133.4, 132.7, 129.5, 128.7, 128.7, 128.4, 128.2, 126.3, 113.9, 113.9, 55.2, 54.7, 52.5, 50.1, 47.0, 46.5, 34.9, 31.5; HRMS (ESI) calcd for C36H38NO6 (M+H)+ 580.2694, found 580.2692.
2-(2-(4-甲氧基苯基)-2-(4-(1-(4-硝基苯基)-3-氧代- 3-苯丙基)氨基)苯基)乙基)丙二酸二甲酯(3k): 无色油状物, 产率64%. 1H NMR (400 MHz, CDCl3) δ: 8.17 (d, J=8.8 Hz, 2H), 7.89 (d, J=7.2 Hz, 2H), 7.59 (dd, J=17.2, 8.0 Hz, 3H), 7.45 (t, J=7.6 Hz, 2H), 7.07 (d, J=8.8 Hz, 2H), 6.93 (d, J=8.4 Hz, 2H), 6.78 (d, J=7.6 Hz, 2H), 6.43 (d, J=8.4 Hz, 2H), 5.04 (t, J=6.4 Hz, 1H), 4.59 (s, 1H), 3.74 (d, J=1.2 Hz, 3H), 3.73~3.69 (m, 1H), 3.67 (d, J=2.0 Hz, 3H), 3.66 (s, 3H), 3.47 (d, J=6.8 Hz, 2H), 3.23 (td, J=7.2, 2.0 Hz, 1H), 2.52 (td, J=7.6, 1.6 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ: 197.2, 169.8, 169.8, 158.1, 150.8, 147.3, 144.8, 136.3, 135.9, 133.8, 133.7, 128.9, 128.7, 128.6, 128.2, 127.5, 124.1, 114.0, 113.9, 55.2, 54.4, 52.5, 50.1, 47.0, 45.8, 34.8, 34.8; HRMS (ESI) calcd for C35H35N2O8 (M+H)+ 611.2388, found 611.2385.
2-(2-(4-甲氧基苯基)-2-(4-((1-(3-甲氧基苯基)-3-氧代-3-苯丙基)氨基)苯基)乙基)丙二酸二甲酯(3l): 无色油状物, 产率63%. 1H NMR (400 MHz, CDCl3) δ: 7.89 (d, J=7.2 Hz, 2H), 7.54 (d, J=7.4 Hz, 1H), 7.43 (t, J=7.8 Hz, 2H), 7.23 (t, J=8.0 Hz, 1H), 7.07 (d, J=8.4 Hz, 2H), 7.02~6.96 (m, 2H), 6.91 (d, J=8.4 Hz, 2H), 6.80~6.74 (m, 3H), 6.47 (d, J=8.4 Hz, 2H), 4.93~4.86 (m, 1H), 4.47 (s, 1H), 3.82 (dd, J=6.8, 2.4 Hz, 1H), 3.76 (s, 3H), 3.74 (s, 3H), 3.70 (d, J=8.0 Hz, 1H), 3.67 (d, J=2.0 Hz, 3H), 3.65 (s, 3H), 3.46 (dd, J=16.0, 4.8 Hz, 1H), 3.35 (dd, J=16.0, 8.0 Hz, 1H), 3.25 (t, J=6.4 Hz, 1H), 2.52 (t, J=7.2 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ: 198.2, 169.9, 169.9, 160.0, 158.0, 145.6, 145.0, 136.7, 136.2, 133.5, 132.9, 129.9, 128.7, 128.4, 128.2, 118.6, 114.3, 114.0, 113.9, 112.6, 112.2, 55.2, 55.0, 52.5, 50.1, 47.1, 46.4, 34.9, 34.9; HRMS (ESI) calcd for C36H38NO7 (M+H)+ 596.2643, found 596.2645.
2-(2-(4-((1-(3-溴苯基)-3-氧代-3-苯丙基)氨基)苯基)-2-(4-甲氧基苯基)乙基)丙二酸二甲酯(3m): 无色油状物, 产率71%. 1H NMR (400 MHz, CDCl3) δ: 7.72 (d, J=8.4 Hz, 2H), 7.56 (d, J=8.4 Hz, 2H), 7.39 (d, J=7.2 Hz, 2H), 7.30 (t, J=7.6 Hz, 2H), 7.22 (t, J=7.2 Hz, 1H), 7.07 (d, J=8.8 Hz, 2H), 6.91 (d, J=8.4 Hz, 2H), 6.78 (d, J=8.2 Hz, 2H), 6.47 (d, J=8.2 Hz, 2H), 4.95~4.89 (m, 1H), 4.49 (s, 1H), 3.74 (s, 3H), 3.70 (d, J=5.6 Hz, 1H), 3.66 (d, J=2.0 Hz, 3H), 3.65 (s, 3H), 3.41 (dd, J=16.4, 5.6 Hz, 1H), 3.35 (dd, J=16.0, 7.2 Hz, 1H), 3.25 (t, J=7.2 Hz, 1H), 2.52 (t, J=7.6 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ: 197.3, 169.9, 169.9, 158.0, 145.4, 142.9, 136.2, 136.2, 135.4, 133.0, 132.0, 130.1, 129.7, 128.9, 128.7, 128.7, 128.5, 127.5, 126.3, 114.0, 113.9, 55.2, 54.9, 52.5, 50.1, 47.0, 46.3, 34.9; HRMS (ESI) calcd for C35H35NO6Br (M+H)+ 644.1642, found 644.1645.
2-(2-(4-甲氧基苯基)-2-(4-((3-氧代-3-苯基-1-(邻甲苯基)丙基)氨基)苯基)乙基)丙二酸二甲酯(3n): 无色油状物, 产率86%. 1H NMR (400 MHz, CDCl3) δ: 7.90 (d, J=7.2 Hz, 2H), 7.55 (t, J=7.6 Hz, 1H), 7.43 (t, J=7.6 Hz, 3H), 7.19~7.13 (m, 3H), 7.08 (d, J=8.4 Hz, 2H), 6.91 (d, J=8.4 Hz, 2H), 6.78 (d, J=8.4 Hz, 2H), 6.42 (d, J=8.0 Hz, 2H), 5.18~5.12 (m, 1H), 4.36 (s, 1H), 3.74 (s, 3H), 3.70 (d, J=8.0 Hz, 1H), 3.67 (d, J=2.0 Hz, 3H), 3.65 (d, J=1.6 Hz, 3H), 3.42 (dd, J=16.4, 4.8 Hz, 1H), 3.33 (dd, J=16.4, 8.0 Hz, 1H), 3.26 (t, J=7.2 Hz, 1H), 2.53 (t, J=7.6 Hz, 2H), 2.48 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 198.4, 169.9, 158.0, 145.6, 145.6, 140.8, 136.7, 136.2, 134.8, 133.5, 132.7, 130.8, 128.7, 128.5, 128.2, 127.2, 126.7, 125.5, 113.9, 113.7, 55.2, 52.5, 51.1, 51.1, 50.1, 47.1, 44.6, 34.9, 34.9; HRMS (ESI) calcd for C36H38NO6 (M+H)+ 580.2694, found 580.2691.
2-(2-(2-溴-4-((1-(4-氯苯基)-3-氧代-3-苯丙基)氨基)- 5-甲基苯基)-2-(4-甲氧基苯基)乙基)丙二酸二甲酯(3o): 无色油状物, 产率86%. 1H NMR (400 MHz, CDCl3) δ: 7.88 (d, J=7.2 Hz, 2H), 7.55 (t, J=7.2 Hz, 1H), 7.43 (t, J=7.8 Hz, 2H), 7.39 (d, J=7.2 Hz, 2H), 7.32 (t, J=7.6 Hz, 2H), 7.25~7.21 (m, 1H), 7.11 (d, J=8.8 Hz, 2H), 6.97 (d, J=8.4 Hz, 1H), 6.79~6.72 (m, 3H), 6.46 (dd, J=8.0, 2.0 Hz, 1H), 4.91 (dd, J=7.6, 4.8 Hz, 1H), 4.60 (s, 1H), 4.27 (t, J=8.0 Hz, 1H), 3.73 (s, 3H), 3.67 (d, J=1.6 Hz, 3H), 3.65 (s, 3H), 3.46 (dd, J=16.4, 4.8 Hz, 1H), 3.37 (dd, J=16.4, 7.6 Hz, 1H), 3.27 (td, J=7.2, 3.2 Hz, 1H), 2.59~2.43 (m, 2H); 13C NMR (101 MHz, CDCl3) δ: 198.1, 169.8, 169.7, 158.1, 146.5, 142.5, 136.6, 135.1, 133.6, 131.1, 123.0, 128.9, 128.7, 128.6, 128.2, 127.6, 126.3, 125.6, 117.7, 113.8, 113.4, 55.2, 54.7, 52.5, 50.0, 46.2, 45.2, 34.7, 34.6; HRMS (ESI) calcd for C35H35NO6Br (M+H)+ 644.1642, found 644.1645.
2-(2-(4-甲氧基苯基)-2-(2-甲基-4-)((3-氧代-1,3-二苯丙基)氨基)苯基)乙基)丙二酸二甲酯(3p): 无色油状物, 产率86%. 1H NMR (400 MHz, CDCl3) δ: 7.90 (d, J=9.6 Hz, 2H), 7.58~7.53 (m, 1H), 7.47~7.40 (m, 4H), 7.32 (t, J=7.6 Hz, 2H), 7.23 (t, J=7.2 Hz, 1H), 7.04 (d, J=8.8 Hz, 2H), 6.98 (d, J=8.0 Hz, 1H), 6.76 (d, J=8.8 Hz, 2H), 6.38 (dd, J=7.6, 2.8 Hz, 2H), 4.98~4.92 (m, 1H), 4.36 (s, 1H), 3.92 (t, J=8.0 Hz, 1H), 3.74 (s, 3H), 3.67 (d, J=3.6 Hz, 3H), 3.66 (s, 3H), 3.48 (dd, J=16.0, 5.2 Hz, 1H), 3.38 (dd, J=16.0, 7.6 Hz, 1H), 3.33~3.27 (m, 1H), 2.48 (t, J=7.6 Hz, 2H), 2.07 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 198.3, 169.9, 157.9, 145.3, 143.3, 137.2, 136.7, 135.8, 133.4, 130.8, 129.0, 128.8, 128.7, 128.2, 127.3, 127.1, 126.4, 116.4, 113.8, 111.3, 55.2, 54.9, 52.5, 50.1, 46.4, 42.7, 35.1, 19.9; HRMS (ESI) calcd for C36H38NO6 (M+H)+ 580.2694, found 580.2696.
2-(2-(2-溴-4-((1-(4-氯苯基)-3-氧代-3-苯丙基)氨基)苯基)-2-(4-甲氧基苯基)乙基)丙二酸二甲酯(3q): 无色油状物, 产率86%. 1H NMR (400 MHz, CDCl3) δ: 7.88 (d, J=8.0 Hz, 2H), 7.57 (t, J=7.2 Hz, 1H), 7.44 (t, J=7.8 Hz, 2H), 7.34 (d, J=8.4 Hz, 2H), 7.28 (d, J=8.4 Hz, 2H), 7.12 (d, J=8.4 Hz, 2H), 6.99 (s, 1H), 6.80~6.75 (m, 2H), 6.72 (dd, J=2.4, 1.6 Hz, 1H), 6.43 (dd, J=8.4, 2.4 Hz, 1H), 4.88 (dd, J=7.2, 5.2 Hz, 1H), 4.62 (s, 1H), 4.28 (t, J=8.0 Hz, 1H), 3.74 (s, 3H), 3.69~3.64 (m, 6H), 3.46~3.32 (m, 2H), 3.27 (td, J=7.2, 2.8 Hz, 1H), 2.61~2.41 (m, 2H); 13C NMR (101 MHz, CDCl3) δ: 197.7, 169.7, 169.7, 158.1, 146.2, 141.0, 136.4, 134.9, 133.7, 133.2, 131.4, 129.1, 128.9, 128.9, 128.6, 128.6, 128.2, 125.6, 117.8, 113.8, 113.4, 55.2, 54.1, 52.6, 49.9, 46.0, 45.2, 34.7, 34.6; HRMS (ESI) calcd for C35H34BrClNO6 (M+H)+ 678.1253, found 678.1255.
2-(2-(2-((1-(4-氯苯基)-3-氧代-3-苯丙基)氨基) -5-甲基苯基)-2-(4-甲氧基苯基)乙基)丙二酸二甲酯(3r): 无色油状物, 产率40%. 1H NMR (400 MHz, CDCl3) δ: 7.95~7.88 (m, 1H), 7.88~7.83 (m, 1H), 7.54 (q, J=7.6 Hz, 1H), 7.43 (dt, J=12.0, 7.6 Hz, 2H), 7.37 (d, J=7.2 Hz, 1H), 7.29 (t, J=7.6 Hz, 1H), 7.23~7.17 (m, 1H), 7.15 (dd, J=8.4, 4.4 Hz, 3H), 7.08~6.99 (m, 1H), 6.86 (d, J=8.8 Hz, 1H), 6.84~6.74 (m, 3H), 6.37 (dd, J=18.0, 8.4 Hz, 1H), 5.03~4.96 (m, 1H), 4.64 (s, 1H), 3.95~3.86 (m, 1H), 3.78 (d, J=11.6 Hz, 3H), 3.68 (d, J=21.6 Hz, 3H), 3.62 (d, J=4.4 Hz, 3H), 3.47 (td, J=16.4, 8.0 Hz, 1H), 3.40~3.19 (m, 2H), 2.71~2.61 (m, 1H), 2.51~2.40 (m, 1H), 2.15 (d, J=8.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 203.1, 202.9, 175.7, 175.5, 175.3, 175.2, 163.9, 163.8, 148.9, 148.7, 147.5, 142.5, 142.4, 139.0, 138.9, 138.5, 135.1, 134.9, 134.1, 133.9, 134.0, 133.9, 133.6, 133.6, 133.4, 133.2, 133.1, 132.9, 132.5, 132.4, 131.9, 131.6, 131.5, 119.6, 119.6, 118.3, 118.1, 60.7, 60.6, 59.9, 59.7, 57.9, 57.9, 55.2, 55.1, 52.7, 52.3, 47.8, 47.7, 38.9, 38.6, 26.0, 26.0; HRMS (ESI) calcd for C36H37ClNO6 (M+H)+ 614.2304, found 614.2305.
2-(2-(2-甲氧基苯基)-2-(4-((3-氧代-1,3-二苯基丙基)氨基)苯基)乙基)丙二酸二甲酯(3s): 无色油状物, 产率37%. 1H NMR (400 MHz, CDCl3) δ: 7.89 (d, J=7.2 Hz, 2H), 7.54 (t, J=7.2 Hz, 1H), 7.45~7.39 (m, 4H), 7.30 (t, J=7.6 Hz, 2H), 7.22 (t, J=7.2 Hz, 1H), 7.12 (t, J=7.8 Hz, 2H), 6.96 (d, J=8.4 Hz, 2H), 6.85 (t, J=7.6 Hz, 1H), 6.78 (d, J=7.6 Hz, 1H), 6.46 (d, J=8.4 Hz, 2H), 4.96~4.91 (m, 1H), 4.43 (s, 1H), 4.25 (t, J=8.0 Hz, 1H), 3.72 (d, J=1.6 Hz, 3H), 3.65 (d, J=1.6 Hz, 3H), 3.61 (d, J=1.6 Hz, 3H), 3.46 (dd, J=15.6, 4.8 Hz, 1H), 3.37 (dd, J=16.0, 7.8 Hz, 1H), 3.28~3.22 (m, 1H), 2.59~2.45 (m, 2H); 13C NMR (101 MHz, CDCl3) δ: 198.3, 170.0, 169.9, 169.9, 156.9, 145.4, 143.2, 136.7, 133.4, 132.5, 132.4, 128.8, 128.7, 128.2, 127.7, 127.4, 127.2, 126.4, 120.6, 113.8, 110.7, 55.4, 55.0, 52.4, 50.3, 46.4, 40.0, 34.0; HRMS (ESI) calcd for C35H36NO6 (M+H)+ 566.2537, found 566.2538.
2-(2-(3,4-二甲氧基苯基)-2-(4-((3-氧代-1,3-二苯基丙基)氨基)苯基)乙基)丙二酸二甲酯(3t): 白色固体, m.p.110.2~118.0, 产率68%. 1H NMR (400 MHz, CDCl3) δ: 7.88 (d, J=7.2 Hz, 2H), 7.54 (t, J=7.6 Hz, 1H), 7.42 (dt, J=7.6, 4.0 Hz, 4H), 7.30 (t, J=7.6 Hz, 2H), 7.21 (t, J=7.2 Hz, 1H), 6.92 (d, J=8.4 Hz, 2H), 6.76~6.69 (m, 2H), 6.65 (t, J=2.0 Hz, 1H), 6.48 (d, J=7.6 Hz, 2H), 4.95 (dd, J=7.7, 5.1 Hz, 1H), 4.50 (s, 1H), 3.81 (s, 3H), 3.79 (d, J=2.4 Hz, 3H), 3.71 (t, J=9.1 Hz, 1H), 3.66 (d, J=2.4 Hz, 3H), 3.65 (d, J=1.2 Hz, 3H), 3.46 (dd, J=16.0, 5.2 Hz, 1H), 3.38 (dd, J=16.0, 7.6 Hz, 1H), 3.26 (td, J=7.2, 2.0 Hz, 1H), 2.57~2.47 (m, 2H); 13C NMR (101 MHz, CDCl3) δ: 198.2, 169.9, 169.9, 148.8, 147.5, 145.7, 143.1, 136.7, 133.5, 132.7, 128.8, 128.7, 128.4, 128.2, 127.4, 126.4, 119.6, 113.9, 111.3, 111.2, 55.9, 55.8, 54.9, 52.5, 50.1, 47.5, 46.4, 34.9, 34.8; HRMS (ESI) calcd for C36H38NO7 (M+H)+ 596.2643, found 596.2645.
2-(2-(2,3,4-二甲氧基苯基)-2-(4-((3-氧代-1,3-二苯基丙基)氨基)苯基)乙基)丙二酸二甲酯(3u): 无色油状物, 产率72%. 1H NMR (400 MHz, CDCl3) δ: 7.88 (d, J=7.2 Hz, 2H), 7.54 (t, J=7.2 Hz, 1H), 7.44~7.38 (m, 4H), 7.32~7.27 (m, 2H), 7.21 (t, J=6.8 Hz, 1H), 6.94 (d, J=8.4 Hz, 2H), 6.87 (dd, J=8.8, 2.0 Hz, 1H), 6.59 (dd, J=8.8, 1.6 Hz, 1H), 6.47 (d, J=8.4 Hz, 2H), 4.94 (ddd, J=7.2, 5.2, 1.2 Hz, 1H), 4.46 (s, 1H), 4.11 (t, J=8.0 Hz, 1H), 3.80 (s, 3H), 3.80 (s, 3H), 3.67 (d, J=2.0 Hz, 3H), 3.63 (s, 3H), 3.60 (d, J=4.0 Hz, 3H), 3.49~3.35 (m, 2H), 3.28 (t, J=7.2 Hz, 1H), 2.55~2.42 (m, 2H); 13C NMR (101 MHz, CDCl3) δ: 198.3, 167.0, 169.9, 152.1, 151.7, 145.4, 143.1, 142.3, 136.7, 133.4, 132.8, 130.2, 128.8, 128.7, 128.2, 127.3, 126.4, 121.7, 114.0, 113.9, 107.1, 60.7, 60.6, 55.9, 55.0, 52.5, 52.4, 50.2, 46.4, 40.4, 40.4, 34.4; HRMS (ESI) calcd for C37H40NO8 (M+H)+ 626.2748, found 626.2745.
2-(2-(4-甲氧基苯基)-2-(4-((3-氧代-1-苯基-3-噻吩基)丙基)氨基)苯基)乙基)丙二酸二甲酯(3v): 无色油状物, 产率80%.1H NMR (400 MHz, CDCl3) δ: 7.95 (d, J=1.6 Hz, 1H), 7.48 (d, J=5.2 Hz, 1H), 7.40 (d, J=7.2 Hz, 2H), 7.33~7.26 (m, 3H), 7.22 (t, J=7.2 Hz, 1H), 7.07 (d, J=8.8 Hz, 2H), 6.91 (d, J=8.6 Hz, 2H), 6.77 (d, J=8.4 Hz, 2H), 6.46 (d, J=8.4 Hz, 2H), 4.92~4.87 (m, 1H), 4.52 (s, 1H), 3.73 (s, 3H), 3.69 (s, 1H), 3.66 (d, J=2.4 Hz, 3H), 3.64 (d, J=1.2 Hz, 3H), 3.34 (dd, J=15.6, 5.2 Hz, 1H), 3.28 (dd, J=7.2, 4.4 Hz, 1H), 3.24 (td, J=7.4, 2.7 Hz, 1H), 2.52 (td, J=7.7, 1.8 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ: 192.4, 169.9, 158.0, 145.6, 143.1, 142.1, 136.2, 132.8, 132.6, 128.9, 128.7, 128.4, 127.4, 126.9, 126.7, 126.3, 113.9, 113.9, 55.2, 55.2, 52.5, 50.1, 47.7, 47.0, 34.9, 34.9; HRMS (ESI) calcd for C33H34NO6S (M+H)+ 572.2102, found 572.2104.
2-(2-(4-甲氧基苯基)-2-(4-((3-氧代-1-苯基-3-甲基基)丙基)氨基)苯基)乙基)丙二酸二甲酯(3w): 无色油状物, 产率98%. 1H NMR (400 MHz, CDCl3) δ: 7.35~7.28 (m, 4H), 7.22 (t, J=7.2 Hz, 1H), 7.08 (d, J=8.4 Hz, 2H), 6.92 (d, J=8.4 Hz, 2H), 6.78 (d, J=7.6 Hz, 2H), 6.46 (d, J=8.4 Hz, 2H), 4.77 (t, J=6.6 Hz, 1H), 4.36 (s, 1H), 3.74 (s, 3H), 3.69 (s, 1H), 3.66 (d, J=2.8 Hz, 3H), 3.65 (d, J=1.6 Hz, 3H), 3.25 (td, J=7.2, 2.0 Hz, 1H), 2.88 (d, J=6.4 Hz, 2H), 2.52 (t, J=7.6 Hz, 2H), 2.08 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 207.1, 169.9, 169.8, 158.0, 145.4, 142.7, 136.2, 132.9, 128.8, 128.7, 128.5, 127.4, 126.3, 113.9, 55.2, 54.5, 52.5, 51.3, 50.1, 47.1, 34.9, 34.9, 30.7; HRMS (ESI) calcd for C30H34NO6 (M+H)+ 504.2381, found 504.2388.