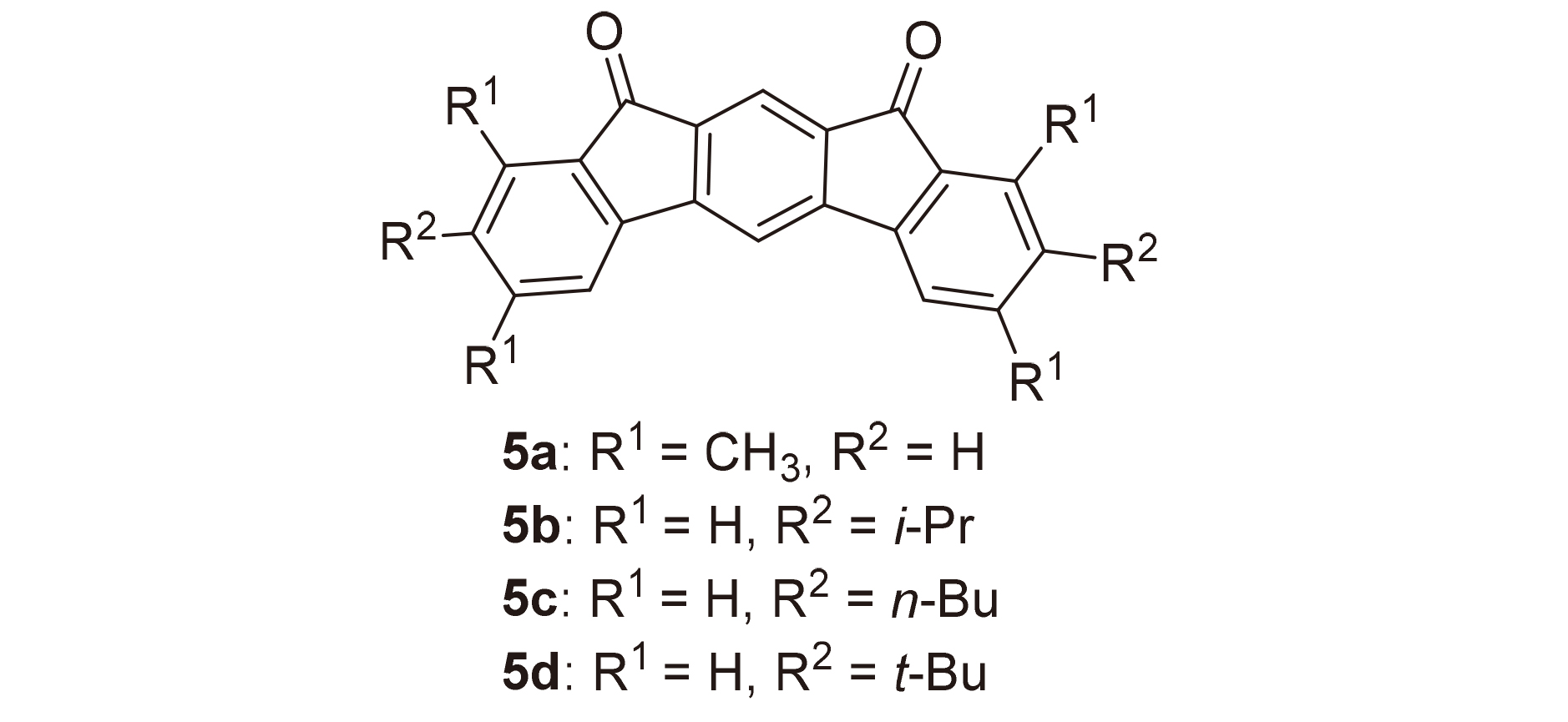
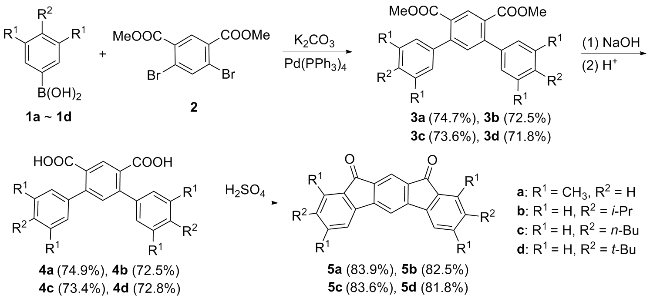
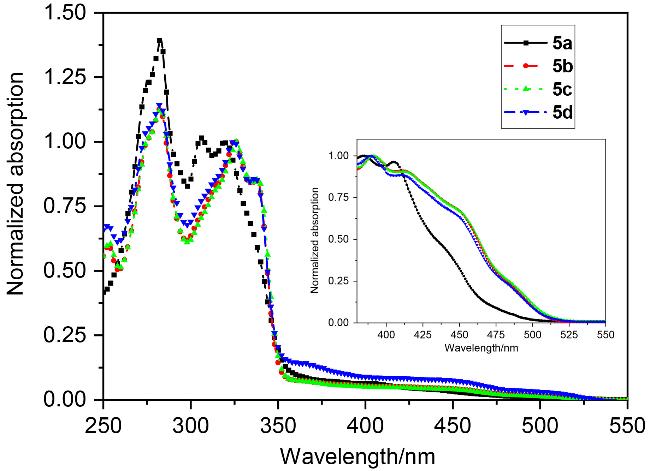
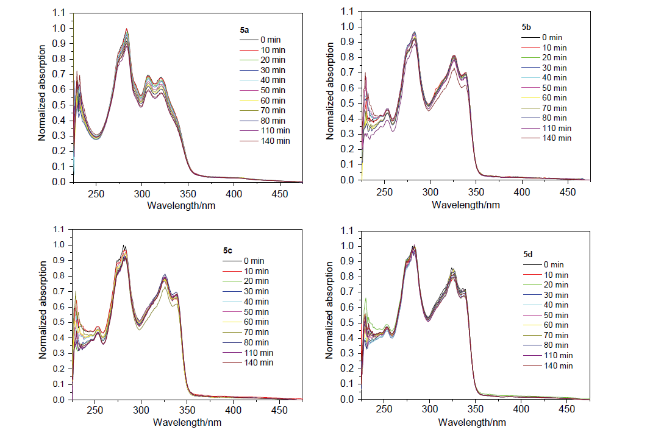
在100 mL三口烧瓶中, 加入4,6-二溴间苯二甲酸二甲酯(1.06 g, 3 mmol)、3,5-二甲基苯硼酸(1.8 g, 12 mmol)和碳酸钾(1.66 g, 12 mmol)饱和水溶液, 抽真空通氩气, 在氩气的保护下加入催化剂Pd(PPh3)4 (139 mg, 0.12 mmol)和50 mL无氧四氢呋喃(THF), 搅拌并控制反应温度为70 ℃, 薄板监测反应, 待原料反应完毕后, 停止反应. 冷却反应体系至室温, 反应液倾倒入饱和NH4Cl溶液中, 用二氯甲烷(10 mL×3)萃取有机层, 合并有机层并用无水硫酸镁干燥, 过滤, 浓缩, 用石油醚和乙酸乙酯的混合溶剂(V∶V=60∶1、40∶1)作梯度洗脱剂来过柱分离提纯, 得到1.02 g白色固体3a, 产率85%. m.p. 80~82 ℃; 1H NMR (CDCl3, 400 MHz) δ: 2.34 (s, 12H), 3.70 (s, 6H), 6.95 (s, 4H), 7.01 (s, 2H), 7.39 (s, 1H), 8.27 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ: 21.5, 52.3, 126.2, 129.4, 129.6, 131.8, 133.5, 137.8, 140.3, 145.6, 168.3; IR (KBr) v: 3017, 2914, 1725, 1601, 1545, 1433, 1375 cm-1; MS m/z (%): 402. Anal. calcd for C26H26O4: C 77.59, H 6.51, O 15.90; found C 77.63, H 6.52.
4,6-二(4-异丙基苯基)间苯二甲酸二甲酯(3b): 961.0 mg, 白色固体, 产率74%. m.p. 139~141 ℃; 1H NMR (CDCl3, 400 MHz) δ: 1.28 (d, J=8 Hz, 12H), 2.92~2.98 (m, 2H), 3.71 (s, 6H), 7.24~7.29 (m, 8H), 7.42 (s, 1H), 8.32 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ: 24.1, 34.0, 52.3, 126.4, 128.4, 129.2, 132.1, 133.7, 137.6, 145.5, 148.7, 168.3; IR (KBr) v: 3032, 2960, 2867, 1721, 1610, 1513, 1430, 1290, 1253 cm-1; MS m/z (%): 430. Anal. calcd for C28H30O4 C 78.11, H 7.02, O 14.86; found C 78.15, H 7.05.
4,6-二(4-正丁基苯基)间苯二甲酸二甲酯(3c): 986.5 mg, 白色固体, 产率72%. m.p. 70~72 ℃; 1H NMR (CDCl3, 400 MHz) δ: 0.94 (t, J=8 Hz, 6H), 1.33~1.42 (m, 4H), 1.59~1.67 (m, 4H), 2.65 (t, J=8 Hz, 4H), 3.70 (s, 6H), 7.20~7.25 (m, 8H), 7.41 (s, 1H), 8.31 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ: 14.1, 22.5, 33.6, 35.5, 52.3, 128.3, 128.4, 129.2, 132.1, 133.6, 137.5, 142.8, 145.5, 168.3; IR (KBr) v: 3023, 2957, 2856, 1717, 1613, 1518, 1433, 1290, 1260 cm-1; MS m/z (%): 458. Anal. calcd for C30H34O4 C 78.57, H 7.47, O 13.95; found C 78.60, H 7.45.
4,6-二(4-叔丁基苯基)间苯二甲酸二甲酯(3d): 970.1 mg, 白色固体3d, 产率71%. m.p. 196~197 ℃; 1H NMR (CDCl3, 400 MHz) δ: 1.35 (s, 18H), 3.71 (s, 6H), 7.28 (d, J=8 Hz, 4H), 7.42 (d, J=8 Hz, 4H), 7.43 (s, 1H), 8.32 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ: 31.5, 34.8, 52.3, 125.2, 128.2, 129.2, 132.1, 133.8, 137.3, 145.4, 150.9, 168.3; IR (KBr) v: 3035, 2960, 2867, 1721, 1606, 1516, 1430, 1360, 1280, 1254 cm-1; MS m/z (%): 458. Anal. calcd for C30H34O4 C 78.57, H 7.47, O 13.95; found C 78.55, H 7.50.
在100 mL单口圆底烧瓶中加入化合物3a (643.2 mg 1.6 mmol), NaOH (256 mg 6.4 mmol), 40 mL乙醇和4 mL水, 加热回流过夜, 减压蒸去一半溶剂后加入稀盐酸酸化, 生成白色固体, 抽滤, 干燥, 得到454.2 mg白色固体4a, 产率75%. m.p. 309~310 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 2.30 (s, 12H), 7.01 (s, 6H), 7.28 (s, 1H), 8.02 (s, 1H), 13.01 (s, 2H); 13C NMR (DMSO-d6, 100 MHz) δ: 21.0, 126.2, 129.2, 130.2, 130.7, 132.7, 137.2, 139.9, 143.4, 168.9; IR (KBr) v: 3440, 2907, 1708, 1685, 1602, 1436, 1375 cm-1; MS m/z (%): 374. Anal. calcd for C24H22O4: C 76.99, H 5.92, O 17.09; found C 77.01, H 5.95.
4,6-二(4-异丙基苯基)间苯二甲酸(4b): 402.3 mg, 白色固体, 产率62%. m.p. 219~221 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 1.22 (d, J=8 Hz, 12H), 2.89~2.95 (m, 2H), 7.27~7.35 (m, 9H), 8.01 (s, 1H); 13C NMR (DMSO-d6, 100 MHz) δ: 24.0, 33.3, 126.3, 128.5, 130.3, 130.8, 132.8, 137.3, 143.1, 148.0, 168.9; IR (KBr) v: 2969, 2872, 2641, 1701, 1595, 1466, 1410, 1298, 1252 cm-1; MS m/z (%): 402. Anal. calcd for C26H26O4 C 77.59, H 6.51, O 15.90; found C 77.62, H 6.53.
4,6-二(4-正丁基苯基)间苯二甲酸(4c): 436.2 mg, 白色固体, 产率63%. m.p.>300 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 0.91 (t, J=8 Hz, 6H), 1.29~1.38 (m, 4H), 1.54~1.62 (m, 4H), 2.62 (t, J=8 Hz, 4H), 7.24 (d, J=8 Hz, 4H), 7.32 (s, 1H), 7.33 (d, J=8 Hz, 4H), 8.05 (s, 1H), 13.00 (s, 2H); 13C NMR (DMSO-d6, 100 MHz) δ: 13.9, 21.9, 33.1, 34.6, 128.2, 128.4, 130.4, 130.6, 132.8, 137.2, 142.0, 143.2, 168.9; IR (KBr) v: 2963, 2928, 2848, 2637, 1696, 1602, 1486, 1412, 1264 cm-1; MS m/z (%): 430. Anal. calcd for C28H30O4 C 78.11, H 7.02, O 14.86; found C 78.13, H 7.01.
4,6-二(4-叔丁基苯基)间苯二甲酸(4d): 504.9 mg, 白色固体, 产率73%. m.p. 291~293 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 1.31 (s, 18H), 7.34 (s, 1H), 7.36 (d, J=8 Hz, 4H), 7.45 (d, J=8 Hz, 4H), 8.03 (s, 1H); 13C NMR (DMSO-d6, 100 MHz) δ: 31.2, 34.4, 125.2, 128.2, 130.3, 130.8, 132.8, 136.9, 143.0, 150.2, 168.9; IR (KBr) v: 2962, 2865, 2510, 1714, 1602, 1463, 1400, 1362, 1298, 1268 cm-1; MS m/z (%): 430. Anal. calcd for C28H30O4 C 78.11, H 7.02, O 14.86; found C 78.15, H 7.05.
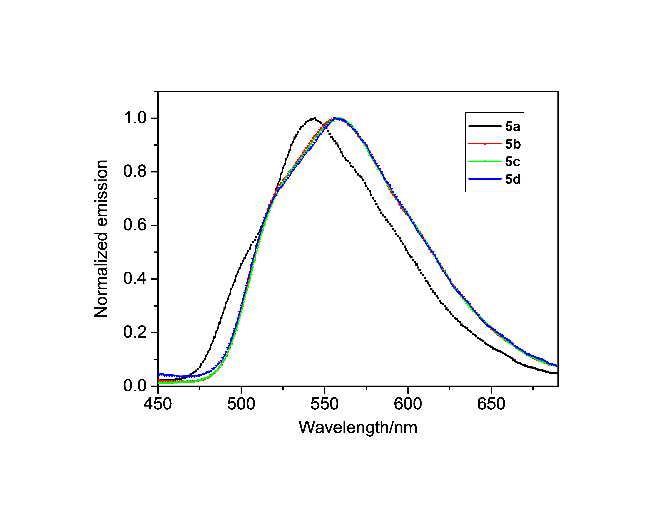
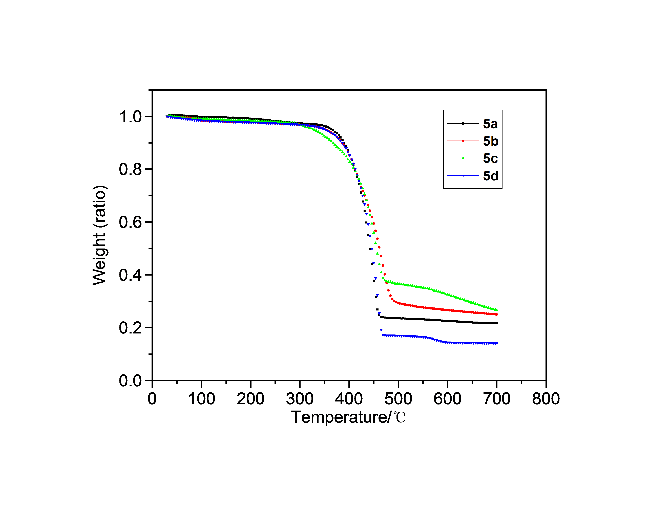
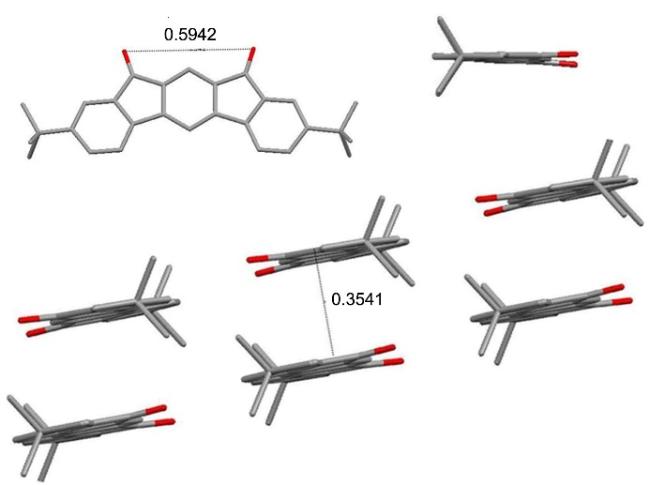
在50 mL单口圆底烧瓶中加入化合物4a (411.4 mg, 1.1 mmol)和14.9 mL浓硫酸, 在室温下搅拌3 h后倒入冰水中, 抽滤并用水洗涤, 然后将固体倒入碳酸钾溶液中搅拌数小时, 抽滤, 用水洗至中性, 并用真空干燥箱干燥, 得到304.5 mg黄色固体5a, 产率82%. m.p. 377~379 ℃; 1H NMR (CDCl3, 400 MHz) δ: 2.42 (s, 6H), 2.60 (s, 6H), 6.94 (s, 2H), 7.27 (s, 2H), 7.61 (s, 1H), 7.84 (s, 1H); IR (KBr) v: 3020, 2914, 2856, 1707, 1607, 1588, 1452, 1380, 1290 cm-1; MS (APCI) calcd for C24H18O2 338.13, found 339.14 [M+H]+. Anal. calcd for C24H18O2 C 85.18, H 5.36, O 9.46; found C 85.15, H 5.32.
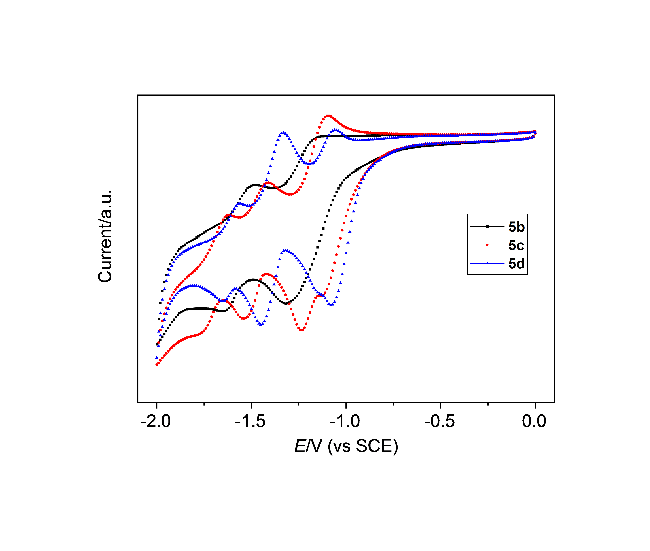
3,9-二异丙基茚并芴-5,7-二酮(5b): 291 mg, 黄色固体, 产率72%. m.p. 236~237 ℃; 1H NMR (CDCl3, 400 MHz) δ: 1.28 (d, J=8 Hz, 12H), 2.92~2.99 (m, 2H) 7.39 (d, J=8 Hz, 2H), 7.50 (d, J=8 Hz, 2H), 7.55~7.56 (m, 3H), 7.83 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ: 23.8, 34.4, 111.9, 120.1, 121.1, 122.6, 133.0, 134.5, 135.4, 140.7, 152.0, 152.1, 192.3; IR (KBr) v: 3071, 2956, 2867, 1708, 1618, 1596, 1463, 1384, 1319, 1236 cm-1; MS (APCI) calcd for C26H22O2 [M+H]+ 366.16, found 367.17. Anal. calcd for C26H22O2 C 85.22, H 6.05, O 8.73; found C 85.25, H 6.01.
3,9-二正丁基茚并芴-5,7-二酮(5c): 318.5 mg, 黄色固体, 产率73%. m.p. 269~270 ℃; 1H NMR (CDCl3, 400 MHz) δ: 0.94 (t, J=8 Hz, 6H), 1.32~1.41 (m, 4H), 1.57~1.65 (m, 4H), 2.62 (t, J=8 Hz, 4H), 7.32 (d, J=8 Hz, 2H), 7.44~7.46 (m, 4H), 7.49 (s, 1H), 7.78 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ: 14.0, 22.4, 33.4, 35.7, 111.8, 120.0, 121.0, 124.5, 134.4, 134.8, 135.3, 140.5, 146.1, 151.9, 192.2; IR (KBr) v: 3060, 2956, 2928, 2852, 1708, 1621, 1604, 1452, 1376, 1232 cm-1; MS (APCI) calcd for C28H26O2 [M+H]+ 394.19, found 395.20. Anal. calcd for C28H26O2 C 85.25, H 6.64, O 8.11; found C 85.27, H 6.67.
3,9-二叔丁基茚并芴-5,7-二酮(5d): 362.3 mg, 黄色固体, 产率83%. m.p. 268~269 ℃; 1H NMR (CDCl3, 400 MHz) δ: 1.37 (s, 18H), 7.57~7.59 (m, 4H), 7.65 (s, 1H), 7.77 (s, 2H), 7.91 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ: 31.3, 35.4, 112.0, 120.3, 120.9, 121.9, 131.7, 134.7, 135.3, 140.4, 151.9, 154.6, 192.5; IR (KBr) v: 3043, 2974, 2918, 2902, 2867, 1722, 1600, 1484, 1464, 1362, 1232 cm-1; MS (APCI) calcd for C28H26O2 [M+H]+ 394.19, found 395.20. Anal. calcd for C28H26O2 C 85.25, H 6.64, O 8.11; found C 85.22, H 6.60.