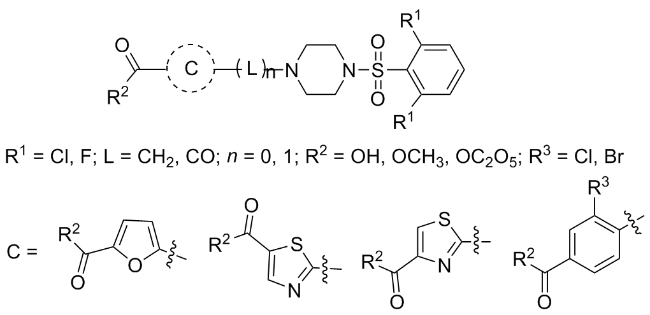
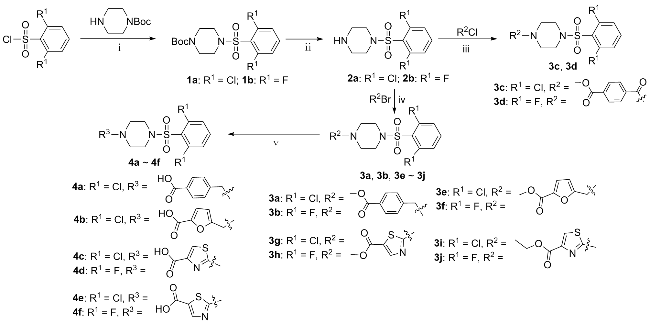
将4-溴甲基苯甲酸甲酯(0.52 g, 2.26 mmol)溶于5 mL N,N-二甲基甲酰胺(DMF)中, 加入Cs2CO3 (1.47 g, 4.52 mmol), 再将1-(2,6-二氯苯磺酰基)哌嗪(0.83 g, 3.39 mmol)加入至反应液中, 将温度升至120 ℃, TLC监测反应进程. 反应完全后, 向反应液中加入20 mL蒸馏水, 用乙酸乙酯萃取(20 mL×3), 依次用蒸馏水、饱和食盐水洗涤, 有机相用无水MgSO4干燥. 抽滤, 滤液减压浓缩, 柱层析分离提纯, 得到0.76 g白色粉末固体3a, 收率为75.6%. 以同样的方法合成化合物3b, 3e~3j.
4-[6-二氯苯磺酰基)-1-哌嗪甲基]苯甲酸甲酯(3a): m.p. 226~227 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.02 (d, J=6.2 Hz, 2H), 7.66 (s, 2H), 7.44 (d, J=7.8 Hz, 2H), 7.33 (t, J=7.9 Hz, 1H), 4.11 (s, 2H), 3.89 (s, 3H), 3.78 (s, 4H), 3.02 (s, 4H); 13C NMR (101 MHz, CDCl3) δ: 166.14, 135.49, 134.24, 133.30, 132.02, 131.94, 131.72, 130.51, 60.63, 52.49, 51.71, 42.86; HRMS calcd for C19H21Cl2N2O4S [M+H]+ 443.0594, found 443.0593.
4-[6-二氟苯磺酰基)-1-哌嗪甲基]苯甲酸甲酯(3b): 白色粉末固体, 收率为76.3%. m.p. 164~165 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.97 (d, J=7.6 Hz, 2H), 7.58~7.48 (m, 1H), 7.35 (d, J=7.2 Hz, 2H), 7.04 (t, J=8.8 Hz, 2H), 3.91 (s, 3H), 3.56 (s, 2H), 3.28 (s, 4H), 2.55 (s, 4H); 13C NMR (101 MHz, CDCl3) δ: 167.00, 159.93 (dd, J1=259.2 Hz, J2=3.9 Hz), 142.92 (d, J=9.0 Hz), 134.86 (t, J=10.9 Hz), 129.82, 129.41, 129.01, 114.98, 113.35 (dd, J1=24.0 Hz, J2=3.4 Hz), 62.27, 52.36, 52.23, 45.72; HRMS calcd for C19H21F2N2O4S [M+H]+411.1185, found 411.1181.
5-[6-二氯苯磺酰基)-1-哌嗪甲基]-2-呋喃甲酸甲酯(3e): 白色粉末固体, 收率为70.2%. m.p. 104~106 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.46 (s, 1H), 7.44 (s, 1H), 7.33~7.26 (m, 1H), 7.11 (d, J=4.0 Hz, 1H), 6.33 (d, J=4.0 Hz, 1H), 3.87 (s, 3H), 3.62 (s, 2H), 3.43 (s, 4H), 2.56 (s, 4H); 13C NMR (101 MHz, CDCl3) δ: 159.04, 155.63, 144.20, 135.70, 134.70, 132.53, 131.72, 118.80, 111.14, 54.55, 52.34, 51.92, 45.60; HRMS calcd for C17H19Cl2N2O5S [M+H]+ 433.0386, found 433.0386.
5-[6-二氟苯磺酰基)-1-哌嗪甲基]-2-呋喃甲酸甲酯(3f): 白色粉末固体, 收率为85.6%. m.p. 179~180 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.57~7.47 (m, 1H), 7.10 (d, J=3.2 Hz, 1H), 7.02 (t, J=8.8 Hz, 2H), 6.32 (d, J=2.4 Hz, 1H), 3.87 (s, 3H), 3.61 (s, 2H), 3.27 (s, 4H), 2.60 (s, 4H); 13C NMR (101 MHz, CDCl3) δ: 159.94 (dd, J1=259.3 Hz, J2=3.7 Hz), 159.13, 155.74, 144.33, 134.89 (t, J=11.1 Hz), 118.88, 114.88, 113.34 (dd, J1=24.1 Hz, J2=3.5 Hz), 111.23, 54.52, 52.04, 45.66; HRMS calcd for C17H19F2N2O5S [M+H]+ 401.0977, found 401.0977.
2-[6-二氯苯磺酰基)-1-哌嗪基]噻唑-4-甲酸甲酯(3g): 白色粉末固体, 收率为75.3%. m.p. 158~160 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.85 (s, 1H), 7.48 (d, J=8.1 Hz, 2H), 7.35 (t, J=8.0 Hz, 1H), 3.83 (s, 3H), 3.71~3.66 (m, 4H), 3.58~3.53 (m, 4H); 13C NMR (101 MHz, CDCl3) δ: 174.17, 162.33, 147.72, 135.85, 134.58, 133.02, 131.96, 117.55, 52.13, 48.38, 44.96; HRMS calcd for C15H16O4Cl2N3S2 [M+H]+ 435.9954, found 435.9925.
2-[6-二氟苯磺酰基)-1-哌嗪基]噻唑-4-甲酸甲酯(3h): 白色粉末固体, 收率为83.7%. m.p. 198~200 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.83 (s, 1H), 7.59~7.49 (m, 1H), 7.04 (t, J=8.8 Hz, 2H), 3.81 (s, 3H), 3.73~3.68 (m, 4H), 3.43~3.37 (m, 4H); 13C NMR (101 MHz, CDCl3) δ: 174.08, 162.30, 159.82 (dd, J1=259.5 Hz, J2=4.0 Hz), 147.88, 135.27 (t, J=11.0 Hz), 117.61, 115.08 (t, J=16.3 Hz), 113.49 (dd, J1=23.9 Hz, J2=3.5 Hz), 52.08, 47.99, 44.87; HRMS calcd for C15H16O4F2N3S2 [M+H]+ 404.0545, found 404.0540.
2-[6-二氯苯磺酰基)-1-哌嗪基]噻唑-5-甲酸乙酯(3i): 白色粉末固体, 收率为84.3%. m.p. 138~140 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.47 (d, J=5.4 Hz, 3H), 7.34 (t, J=8.0 Hz, 1H), 4.33 (q, J=7.1 Hz, 2H), 3.62 (d, J=4.9 Hz, 4H), 3.54 (d, J=4.8 Hz, 4H), 1.35 (t, J=7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 170.44, 161.65, 144.13, 135.80, 134.66, 132.92, 131.92, 117.25, 61.33, 48.52, 45.02, 14.43; HRMS calcd for C16H17Cl2N3- O4S2Na [M+Na]+ 471.9930, found 471.9942.
2-[6-二氟苯磺酰基)-1-哌嗪基]噻唑-5-甲酸乙酯(3j): 白色粉末固体, 收率为82.6%. m.p. 178~180 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.53 (dq, J=8.4, 5.9 Hz, 1H), 7.47 (s, 1H), 7.04 (t, J=8.7 Hz, 2H), 4.33 (q, J=7.1 Hz, 2H), 3.70~3.63 (m, 4H), 3.43~3.36 (m, 4H), 1.35 (t, J=7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 170.25, 161.60, 159.83 (dd, J1=260.6 Hz, J2=4.0 Hz), 144.11, 135.17 (t, J=11.1 Hz), 117.28, 114.62, 113.46 (dd, J1=24.1 Hz, J2=3.5 Hz), 61.34, 48.23, 44.97, 14.42; HRMS calcd for C16H17F2N3O4S2Na [M+Na]+ 440.0521, found 440.0525.
将三乙胺(0.41 mL, 4.89 mmol)加入到1-(2,6-二氯苯磺酰基)哌嗪(0.4 g, 1.63 mmol)的CH2Cl2 (10 mL)溶液中, 然后加入4-氯甲酰基苯甲酸甲酯(0.42 g, 2.12 mmol), 于室温下反应. 反应结束后, 加入10 mL蒸馏水, 用乙酸乙酯萃取(10 mL×3), 合并有机相, 用无水Na2SO4干燥, 经柱层析分离得到0.62 g白色粉末固体3c, 收率为84.3%. 以相同的方法合成化合物3d.
4-(4-((2,6-二氯苯基)磺酰基)哌嗪-1-羰基)苯甲酸甲酯(3c): m.p. 133~134 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.07 (d, J=8.0 Hz, 2H), 7.45 (dd, J=13.4, 8.0 Hz, 4H), 7.38~7.32 (m, 1H), 3.92 (s, 3H), 3.86 (s, 2H), 3.44 (d, J=37.5 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 169.63, 166.26, 139.26, 135.82, 134.54, 132.98, 131.93, 131.72, 130.09, 127.18, 52.52, 47.74, 45.93, 42.37; HRMS calcd for C19H19Cl2N2O5S [M+H]+ 457.0386, found 457.0370.
4-(4-((2,6-二氟苯基)磺酰基)哌嗪-1-羰基)苯甲酸甲酯(3d): 白色粉末固体, 收率为80.8%. m.p. 181~182 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.08 (d, J=8.4 Hz, 2H), 7.61~7.52 (m, 1H), 7.43 (d, J=8.4 Hz, 2H), 7.06 (t, J=8.8 Hz, 2H), 3.94 (s, 5H), 3.53 (s, 2H), 3.37 (s, 2H), 3.24 (s, 2H); 13C NMR (101 MHz, CDCl3) δ: 169.61, 166.25, 159.82 (dd, J1=259.3 Hz, J2=3.9 Hz), 139.09, 135.26 (t, J=11.0 Hz), 131.76, 130.09, 127.21, 115.06 (t, J=16.2 Hz), 113.51 (dd, J1=23.9 Hz, J2=3.3 Hz), 52.55, 47.37, 45.59, 41.81; HRMS calcd for C19H19O5F2N2S [M+H]+ 425.0977, found 425.0954.
将200 mg 4-[6-二氯苯磺酰基)-1-哌嗪甲基]苯甲酸甲酯溶于5 mL THF溶液中, 加入5 mL质量分数为10%的NaOH水溶液, 搅拌5 min后, 向反应瓶中加入5 mL EtOH, 于60 ℃下反应, TLC监测反应进行, 40 min后结束反应. 减压蒸馏除去混合溶剂, 冰浴条件下用1 mol/L HCl的水溶液调节剩余反应液的pH至酸性, 有大量白色固体析出. 减压抽滤, 蒸馏水洗涤滤饼, 滤饼经真空干燥后得到0.17 g白色粉末固体4a, 收率为90.0%. 以相同的方法制备了化合物4b~4f.
4-[6-二氯苯磺酰基)-1-哌嗪甲基]苯甲酸(4a): m.p. 246~247 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 12.17 (s, 1H), 8.02 (d, J=8.0 Hz, 3H), 7.76 (d, J=8.0 Hz, 3H), 7.69~7.65 (m, 1H), 4.46 (s, 2H), 3.92 (s, 4H), 3.14 (s, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 167.36, 135.00, 134.84, 133.82, 132.70, 131.95, 129.99, 51.04, 42.88; HRMS calcd for C18H19Cl2N2O4S [M+H]+ 429.0437, found 429.0453.
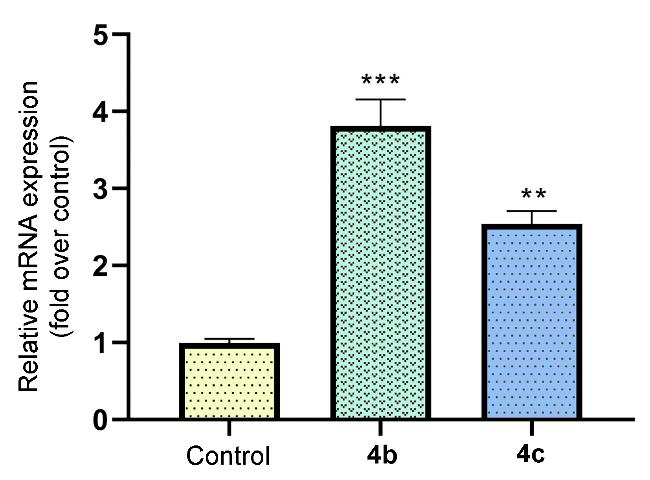
5-[6-二氯苯磺酰基)-1-哌嗪甲基]-2-呋喃甲酸(4b): 白色粉末固体, 收率为76.4%. m.p. 242~243 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.69~7.64 (m, 2H), 7.57 (dd, J=8.8, 7.1 Hz, 1H), 7.12 (d, J=3.3 Hz, 1H), 6.46 (d, J=3.3 Hz, 1H), 3.58 (s, 2H), 3.30 (s, 4H), 2.45 (s, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 159.33, 155.50, 144.60, 134.46, 133.95, 133.84, 132.14, 118.23, 111.20, 53.54, 51.62, 45.26; HRMS calcd for C16H17Cl2N2O5S [M+H]+ 419.0230, found 419.0249.
2-[6-二氯苯磺酰基)-1-哌嗪基]噻唑-5-甲酸(4c):白色粉末固体, 收率为82.1%. m.p. 194~196 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.76 (s, 1H), 7.74 (s, 2H), 7.66 (dd, J=12.0, 7.8 Hz, 1H), 3.58~3.60 (m, 4H), 3.51~3.50 (m, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 170.26, 162.59, 144.14, 134.98, 134.08, 132.66, 48.27, 44.95; HRMS calcd for C14H14Cl2N3O4S2 [M+H]+ 421.9917, found 421.9915.
2-[6-二氟苯磺酰基)-1-哌嗪基]噻唑-5-甲酸(4d): 白色粉末固体, 收率为76.8%. m.p. 235~238 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.89~7.81 (m, 1H), 7.73 (s, 1H), 7.42 (t, J=9.2 Hz, 2H), 3.63~3.61 (m, 4H), 3.35~3.34 (m, 4H); 13C NMR (101 MHz, DMSO-d6) δ 169.46, 162.90, 158.94 (dd, J1=256.9 Hz, J2=3.7 Hz), 146.05, 136.47 (t, J=11.1 Hz), 116.55, 113.83 (dd, J1=23.8 Hz, J2=2.8 Hz), 113.61, 47.46, 44.54; HRMS calcd for C14H14F2N3O4S2 [M+H]+ 390.0388, found 390.0406.
2-[6-二氯苯磺酰基)-1-哌嗪基]噻唑-4-甲酸(4e):白色粉末固体, 收率为72.4%. m.p. 158~160 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.83 (s, 1H), 7.75 (d, J=7.8 Hz, 2H), 7.65 (dd, J=8.8, 7.2 Hz, 1H), 3.67~3.65 (m, 4H), 3.52~3.50 (m, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 174.01, 163.03, 134.98, 134.06, 132.79, 132.65, 118.26, 48.07, 44.87; HRMS calcd for C14H14Cl2- N3O4S2 [M+H]+ 421.9917, found 421.9917.
2-[6-二氟苯磺酰基)-1-哌嗪基]噻唑-4-甲酸(4f):白色粉末固体, 收率为87.5%. m.p. 194~196 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.85 (m, 1H), 7.82 (s, 1H), 7.41 (t, J=9.2 Hz, 2H), 3.72~3.69 (m, 4H), 3.35~3.33 (m, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 173.09, 162.83, 158.94 (dd, J=257.0, 4.0 Hz), 146.26, 136.45 (t, J=11.3 Hz), 119.59, 113.81 (dd, J=23.8, 3.0 Hz), 113.63, 47.21, 44.42; HRMS calcd for C14H14F2N3O4S2 [M+H]+ 390.0428, found 390.0408.
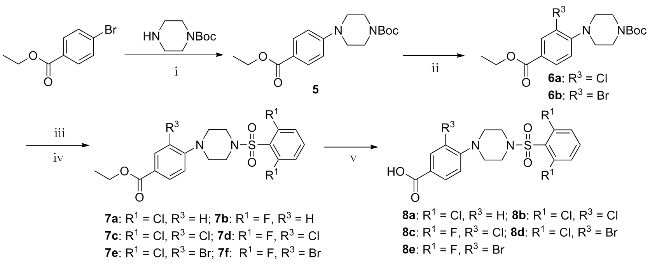
将4-(4-叔丁氧羰基哌嗪)苯甲酸乙酯(5, 1.20 g, 3.58 mmol)溶于10 mL THF中, 加入催化量的浓H2SO4, 将NCS (0.48 g, 3.58 mmol)溶于THF中, 25 ℃下缓慢加入反应液中, 再移至40 ℃反应. TLC进行反应进程的监测, 反应结束后, 减压蒸馏除去THF, 向反应液中加入20 mL蒸馏水, 用乙酸乙酯萃取(20 mL×3), 依次用蒸馏水、饱和食盐水洗涤, 有机层用无水MgSO4干燥. 蒸干, 柱层析分离得到1.16 g白色固体6a, 收率为87.6%. 以相同方法合成化合物6b.
2-氯-4-(4-叔丁氧羰基哌嗪)苯甲酸乙酯的制备(6a): m.p. 80~82 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.03 (d, J=1.8 Hz, 1H), 7.88 (dd, J=8.4, 1.8 Hz, 1H), 7.00 (d, J=8.4 Hz, 1H), 4.34 (q, J=7.2 Hz, 2H), 3.62~3.59 (m, 4H), 3.08~3.05 (m, 4H), 1.48 (s, 9H), 1.37 (t, J=7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 165.43, 154.80, 152.94, 132.14, 129.24, 128.03, 125.70, 119.69, 79.99, 61.09, 50.83, 28.46, 14.36; HRMS calcd for C18H26ClN2O4 [M+H]+ 369.1503, found 369.1516.
2-溴-4-(4-叔丁氧羰基哌嗪)苯甲酸乙酯(6b): 白色固体, 收率为78.3%. m.p. 72~73 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.23 (d, J=1.8 Hz, 1H), 7.93 (dd, J=8.4, 1.8 Hz, 1H), 7.00 (d, J=8.4 Hz, 1H), 4.34 (q, J=7.2 Hz, 2H), 3.63~3.60 (m, 4H), 3.07~3.04 (m, 4H), 1.48 (s, 9H), 1.37 (t, J=7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 165.25, 154.82, 154.34, 135.36, 129.88, 126.28, 120.12, 118.71, 79.97, 61.12, 51.27, 28.46, 14.36; HRMS calcd for C18H26BrN2O4 [M+H]+ 413.1076, found 413.1104.
将4-(4-叔丁氧羰基哌嗪)苯甲酸乙酯(5, 0.54 g, 1.62 mmol)溶于10 mL THF中, 再将吡啶(1.83 mL, 22.73 mmol)和2,6-二氯苯磺酰氯(0.59 mL, 2.42 mmol)分三次加入反应液中, TLC监测反应进程. 反应结束后, 向反应液中加入20 mL蒸馏水, 用乙酸乙酯萃取(20 mL×3), 依次用蒸馏水、饱和食盐水洗涤, 有机相用无水MgSO4干燥. 抽滤, 滤液减压浓缩, 柱层析分离提纯得到淡黄色粉末固体0.52 g, 产率为77.8%.
4-[6-二氯苯磺酰基)-1-哌嗪基]苯甲酸乙酯(7a): m.p. 128~130 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.93 (d, J=8.0 Hz, 2H), 7.48 (d, J=8.0 Hz, 2H), 7.34 (dd, J=8.6, 7.6 Hz, 1H), 6.87 (d, J=9.0 Hz, 2H), 4.32 (q, J=7.2 Hz, 2H), 3.58~3.55 (m, 4H), 3.40~3.38 (m, 4H), 1.36 (t, J=7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 166.52, 153.55, 135.85, 134.66, 132.83, 131.90, 131.34, 121.62, 114.75, 60.63, 48.26, 45.41, 14.52; HRMS calcd for C19H21Cl2N2O4S [M+H]+ 443.0594, found 443.0593.
4-[6-二氟苯磺酰基)-1-哌嗪基]苯甲酸乙酯(7b): 黄色粉末固体, 收率为87.5%. m.p. 208~209 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.96 (d, J=8.8 Hz, 2H), 7.60~7.50 (m, 1H), 7.06 (t, J=8.8 Hz, 2H), 6.96 (d, J=8.8 Hz, 2H), 4.34 (q, J=7.2 Hz, 2H), 3.47 (d, J=7.6 Hz, 8H), 1.37 (t, J=7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 166.54, 159.95 (dd, J1=260.5 Hz, J2=4.1 Hz), 153.41, 135.14 (t, J=11.0 Hz), 131.39, 121.71, 114.78, 113.48 (dd, J1=24.0 Hz, J2=3.5 Hz), 60.70, 47.97, 45.41, 14.56; HRMS calcd for C19H21O4F2N2S [M+H]+ 411.1185 found 411.1165.
3-氯-4-[6-二氯苯磺酰基)-1-哌嗪基]苯甲酸乙酯(7c): 黄色粉末固体, 收率为89.7%. m.p. 178~179 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.03 (d, J=4.0 Hz, 1H), 7.89 (dd, J=8.4, 4.0 Hz, 1H), 7.49 (d, J=8.2 Hz, 2H), 7.36~7.32 (m, 1H), 7.01 (d, J=8.0 Hz, 1H), 4.35 (q, J=7.2 Hz, 2H), 3.62~3.60 (m, 4H), 3.20~3.18 (m, 4H), 1.37 (t, J=7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 165.30, 152.35, 135.78, 134.74, 132.65, 132.13, 131.79, 129.29, 128.11, 126.17, 119.91, 61.16, 50.80, 45.88, 14.34; HRMS calcd for C19H20O4Cl3N2S [M+H]+ 477.0200, found 477.0172.
3-氯-4-[6-二氟苯磺酰基)-1-哌嗪基]苯甲酸乙酯(7d): 黄色粉末固体, 收率为75.2%. m.p. 148~149 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.03 (s, 1H), 7.91 (d, J=8.4 Hz, 1H), 7.61~7.51 (m, 1H), 7.11~7.03 (m, 3H), 4.35 (q, J=7.2 Hz, 2H), 3.48 (s, 4H), 3.28~3.20 (m, 4H), 1.38 (t, J=7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 165.39, 159.97 (dd, J1=259.4 Hz, J2=4.2 Hz), 152.27, 135.03 (t, J=11.0 Hz), 132.24, 129.44, 128.13, 126.36, 120.01, 115.13, 113.46 (dd, J1=24.0 Hz, J2=3.5 Hz), 61.30, 50.54, 45.89, 14.45; HRMS calcd for C19H20Cl- N2SO4F2 [M+H]+ 445.0795, found 445.0794.
3-溴-4-[6-二氯苯磺酰基)-1-哌嗪基]苯甲酸乙酯(7e): 黄色粉末固体, 收率为86.2%. m.p. 168~169 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.22 (d, J=1.8 Hz, 1H), 7.94 (dd, J=8.4, 1.8 Hz, 1H), 7.49 (d, J=8.0 Hz, 2H), 7.36~7.32 (m, 1H), 7.01 (d, J=8.4 Hz, 1H), 4.35 (q, J=7.2 Hz, 2H), 3.63~3.60 (m, 4H), 3.19~3.16 (m, 4H), 1.37 (t, J=7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 165.13, 153.72, 135.77, 135.34, 134.77, 132.64, 131.78, 129.94, 126.74, 120.35, 118.78, 61.19, 51.23, 45.88, 14.34; HRMS calcd for C19H20O4BrCl2N2S [M+H]+ 520.9699, found 520.9688.
3-溴-4-[6-二氟苯磺酰基)-1-哌嗪基]苯甲酸乙酯(7f): 黄色粉末固体, 收率为81.4%. m.p. 120~122 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.21 (d, J=1.8 Hz, 1H), 7.95 (dd, J=8.4, 1.8 Hz, 1H), 7.59~7.52 (m, 1H), 7.09~7.02 (m, 3H), 4.35 (q, J=7.2 Hz, 2H), 3.48 (s, 4H), 3.22~3.20 (m, 4H), 1.37 (t, J=7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 165.18, 159.93 (dd, J1=259.2 Hz, J2=4.0 Hz), 153.63, 135.42, 134.99 (t, J=10.9 Hz), 130.06, 126.91, 120.44, 118.78, 115.28 (t, J=16.7 Hz), 113.44 (dd, J1=23.9 Hz, J2=3.5 Hz), 61.30, 50.97, 45.88, 14.43; HRMS calcd for C19H20O4BrF2N2S [M+H]+ 489.0290, found 489.0275.
4-[6-二氯苯磺酰基)-1-哌嗪基]苯甲酸(8a): 白色粉末固体, 收率为74.6%. m.p. 269~270 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 12.38 (s, 1H), 7.83 (d, J=8.8 Hz, 2H), 7.76 (s, 1H), 7.74 (s, 1H), 7.68~7.64 (m, 1H), 7.03 (t, J=6.6 Hz, 2H), 3.46 (s, 4H), 3.44 (d, J=5.4 Hz, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 167.61, 153.63, 135.04, 134.65, 134.00, 132.72, 131.33, 131.15, 120.85, 114.58, 47.45, 45.39; HRMS calcd for C17H17O4Cl2N2S [M+H]+ 415.0281, found 415.0268.
3-氯-4-[6-二氯苯磺酰基)-1-哌嗪基]苯甲酸(8b): 白色粉末固体, 收率为88.3%. m.p. 238~240 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.91 (s, 1H), 7.82 (d, J=8.0 Hz, 1H), 7.77 (d, J=8.0 Hz, 2H), 7.69~7.65 (m, 1H), 7.10 (d, J=8.0 Hz, 1H), 3.50 (s, 4H), 3.09 (s, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 168.77, 148.95, 137.34, 135.09, 134.63, 134.00, 132.73, 131.50, 129.16, 126.83, 120.17, 51.16, 46.12; HRMS calcd for C17H16O4- Cl3N2S [M+H]+ 448.9891, found 448.9878.
3-氯-4-[6-二氟苯磺酰基)-1-哌嗪基]苯甲酸(8c):白色粉末固体, 收率为78.4%. m.p. 219~220 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.84 (s, 1H), 7.81 (d, J=8.3 Hz, 2H), 7.39 (t, J=9.1 Hz, 2H), 7.17 (d, J=8.4 Hz, 1H), 3.28 (s, 4H), 3.15 (s, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 166.77, 158.96 (dd, J1=257.0 Hz, J2=4.3 Hz), 150.68, 136.51 (t, J=11.2 Hz), 131.10, 129.89, 129.07, 126.70, 120.53, 113.90 (dd, J1=23.8 Hz, J2=2.8 Hz), 113.49, 49.98, 45.55; HRMS calcd for C17H16O4Cl- F2N2S [M+H]+ 417.0482, found 417.0464.
3-溴-4-[6-二氯苯磺酰基)-1-哌嗪基]苯甲酸(8d): 白色粉末固体, 收率为83.8%. m.p. 258~260 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 8.11 (d, J=1.4 Hz, 1H), 7.93 (d, J=8.4 Hz, 1H), 7.77 (d, J=8.0 Hz, 2H), 7.69~7.65 (m, 1H), 7.27 (d, J=8.4 Hz, 1H), 3.52 (s, 4H), 3.17 (s, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 166.50, 153.55, 135.10, 134.88, 134.66, 133.97, 132.74, 130.34, 128.20, 121.72, 118.40, 51.19, 45.99; HRMS calcd for C17H16O4BrCl2N2S [M+H]+ 492.9386, found 492.9373.
3-溴-4-[6-二氟苯磺酰基)-1-哌嗪基]苯甲酸(8e): 白色粉末固体, 收率为87.5%. m.p. 199~200 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 13.10 (s, 1H), 8.10 (d, J=1.8 Hz, 1H), 7.95 (dd, J=8.0, 4.0 Hz, 1H), 7.91~7.86 (m, 1H), 7.45 (t, J=9.2 Hz, 2H), 7.30 (d, J=8.4 Hz, 1H), 3.38~3.36 (m, 4H), 3.23 (d, J=4.2 Hz, 4H); 13C NMR (101 MHz, DMSO-d6) δ: 166.31, 159.50 (dd, J1=257.6 Hz, J2=4.0 Hz), 153.75, 136.99 (t, J=11.0 Hz), 134.90, 130.38, 127.35, 121.80, 118.36, 114.38 (dd, J1=24.2 Hz, J2=3.2 Hz), 114.04, 50.75, 46.00; HRMS calcd for C17H14BrF2N2O4S [M-H]- 458.0031, found 459.0021.