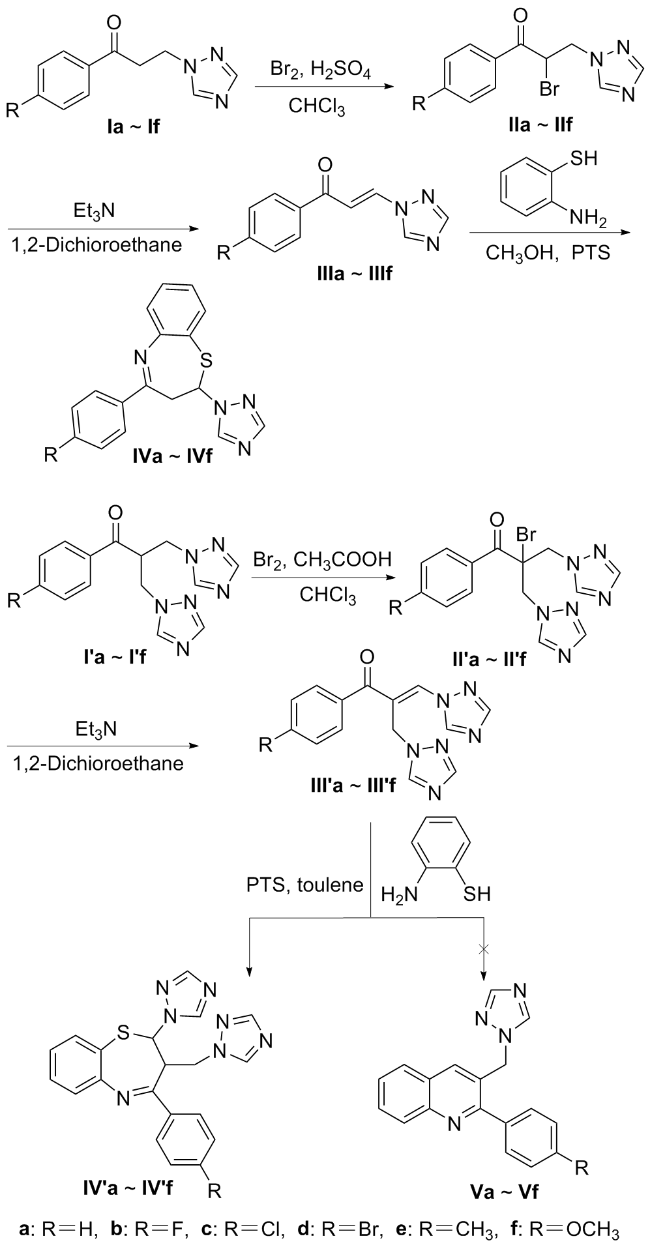
在50 mL的三口瓶中依次加入氯仿(20 mL)、Ⅰa~Ⅰf (8.5 mmol)和浓硫酸(0.5 mL), 加热至回流时滴加溴素 (2.0 g, 12.7 mmol). TLC监控反应至Ⅰa~Ⅰf消失, 停止反应. 反应液分别用饱和碳酸氢钠溶液和水洗涤, 分液. 有机相用无水硫酸钠干燥, 过滤, 减压浓缩溶剂. 剩余物加5 mL石油醚冷冻, 析出固体, 经乙醚和石油醚重结晶得到Ⅱa~Ⅱf.
1-苯基-2-溴-3-(1,2,4-三氮唑)-1-丙酮(Ⅱa): 黄色固体, 产率86%. m.p. 199~200 ℃; 1H NMR (400 MHz, DMSO) δ: 8.65 (s, 1H), 7.59 (s, 1H), 6.79 (d, J=8.0 Hz, 2H), 6.38 (t, J=7.6 Hz, 1H), 6.25 (t, J=7.6 Hz, 2H), 4.77 (t, J=6.8 Hz, 1H), 3.94 (dd, J=14.4, 5.8 Hz, 1H), 3.78 (dd, J=14.4, 7.6 Hz, 1H); 13C NMR (100 MHz, DMSO) δ: 191.4, 143.8, 142.8, 134.2, 133.4, 128.9, 128.7, 53.0, 41.7; ESI-MS m/z: 280.1 [M+H]+. Anal. calcd for C11H10Br- N3O: C 47.17, H 3.60, N 15.00; found C 47.33, H 3.74, N 15.06.
1-(4-氟苯基)-2-溴-3-(1,2,4-三氮唑)-1-丙酮(Ⅱb): 黄色固体, 产率83%. m.p. 137~138 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.21 (s, 1H), 8.03 (m, 2H), 7.96 (s, 1H), 7.18 (t, J=8.6 Hz, 2H), 5.59 (t, J=7.0 Hz, 1H), 5.01 (dd, J=14.4, 7.2 Hz, 1H), 4.72 (dd, J=14.4, 6.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 189.8, 166.4, 153.1, 151.1, 144.4, 131.9, 129.7, 116.2, 51.3, 41.7; ESI-MS m/z: 299.1 [M+H]+. Anal. calcd for C11H9BrFN3O: C 44.32, H 3.04, N 14.10; found C 44.13, H 2.97, N 14.00.
1-(4-氯苯基)-2-溴-3-(1,2,4-三氮唑)-1-丙酮(Ⅱc): 黄色固体, 产率86%. m.p. 110~111 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.28 (s, 1H), 7.99~7.97 (m, 3H), 7.50 (d, J=8.4 Hz, 2H), 5.62 (t, J=6.8 Hz, 1H), 5.04 (dd, J=14.2, 7.0 Hz, 1H), 4.75 (dd, J=14.2, 6.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 190.2, 152.5, 144.6, 141.1, 131.7, 130.4, 129.4, 50.9, 41.6; ESI-MS m/z: 316.0 [M+H]+. Anal. calcd for C11H9BrClN3O: C 42.00, H 2.88, N 13.36; found C 41.73, H 2.99, N 13.41.
1-(4-溴苯基)-2-溴-3-(1,2,4-三氮唑)-1-丙酮(Ⅱd): 黄色固体, 产率86%. m.p. 95~96 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.23 (s, 1H), 7.97 (s, 1H), 7.86 (d, J=8.6 Hz, 2H), 7.66 (d, J=8.6 Hz, 2H), 5.60 (t, J=6.8 Hz, 1H), 5.02 (dd, J=14.2, 7.0 Hz, 1H), 4.73 (dd, J=14.2, 6.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 190.4, 152.6, 144.6, 132.4, 132.1, 130.5, 130.0, 50.9, 41.5; ESI-MS m/z: 359.9 [M+H]+. Anal. calcd for C11H9Br2N3O: C 36.80, H 2.53, N 11.70; found C 36.92, H 2.59, N 11.81.
1-(4-甲基苯基)-2-溴-3-(1,2,4-三氮唑)-1-丙酮(Ⅱe): 黄色固体, 产率74%. m.p. 103~104 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.27 (s, 1H), 7.98 (s, 1H), 7.91 (d, J=8.2 Hz, 2H), 7.31 (d, J=8.0 Hz, 2H), 5.64 (t, J=6.8 Hz, 1H), 5.03 (dd, J=14.2, 7.0 Hz, 1H), 4.74 (dd, J=14.2, 6.8 Hz, 1H), 2.45 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 190.9, 152.2, 145.8, 144.5, 130.9, 129.7, 129.2, 51.1, 41.7, 21.8; ESI-MS m/z: 294.1 [M+H]+. Anal. calcd for C12H12Br- N3O: C 49.00, H 4.11, N 14.29; found C 49.31, H 4.25, N 14.37.
1-(4-甲氧基苯基)-2-溴-3-(1,2,4-三氮唑)-1-丙酮(Ⅱf): 黄色固体, 产率79%. m.p. 88~89 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.24 (s, 1H), 8.03~7.98 (m, 3H), 6.99 (d, J=8.8 Hz, 2H), 5.61 (t, J=7.0 Hz, 1H), 5.03 (dd, J=14.2, 7.0 Hz, 1H), 4.74 (dd, J=14.2, 6.8 Hz, 1H), 3.92 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 189.8, 164.6, 152.3, 144.6, 131.5, 126.3, 114.2, 55.7, 51.2, 41; ESI-MS m/z: 310.2 [M+H]+. Anal. calcd for C12H12BrN3O2: C 46.47, H 3.90, N 13.55; found C 46.60, H 3.98, N 13.67.
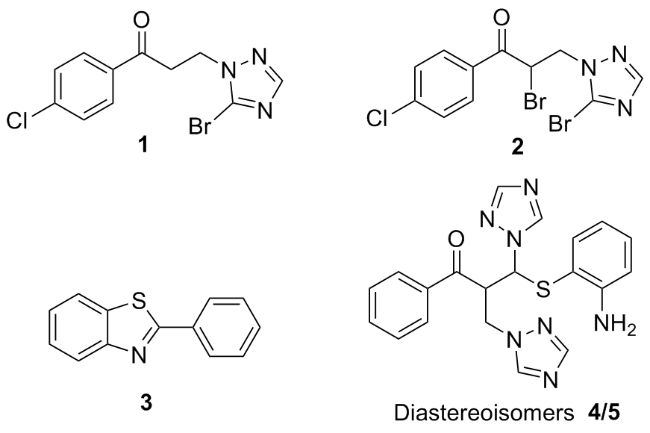
在50 mL的三口瓶中依次加入氯仿(20 mL)、Ⅰc (2.0 g, 8.5 mmol)和浓硫酸(0.5 mL), 加热至回流时滴加溴素 (2.0 g, 12.7 mmol). TLC监控反应至1含量最高时停止反应. 反应液分别用饱和碳酸氢钠溶液和水洗涤, 分液. 有机相用无水硫酸钠干燥、过滤和减压浓缩溶剂, 剩余物经柱层析分离得到3-(5-溴-1,2,4-三氮唑)-1-(4-氯苯基)-1-丙酮(1), 粘稠状液体, 产率10%. 1H NMR (400 MHz, CDCl3) δ: 7.91 (s, 1H), 7.89 (d, J=4.0 Hz, 2H), 7.45 (d, J=8.4 Hz, 2H), 4.61 (t, J=6.8 Hz, 2H), 3.61 (t, J=6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 195.1, 152.7, 140.3, 134.3, 129.5, 129.2, 44.3, 37.4; ESI-MS m/z: 316.1 [M+H]+. Anal. calcd for C11H9BrClN3O: C 42.00, H 2.88, N 13.36; found C 42.12, H 2.93, N 13.45.
在50 mL的三口瓶中依次加入氯仿(20 mL)、Ⅰc (2.0 g, 8.5 mmol)和浓硫酸(0.5 mL), 加热至回流时滴加溴素 (2.0 g, 12.7 mmol). TLC监控反应至Ⅰc消失, 停止反应.反应液分别用饱和碳酸氢钠溶液和水洗涤, 分液. 有机相用无水硫酸钠干燥, 过滤, 减压浓缩溶剂. 剩余物加5 mL石油醚, 冷冻, 析出固体, 过滤, 滤液经柱层析分离得到2-溴-3-(5-溴-1,2,4-三氮唑)-1-(4-氯苯基)-1-丙酮(2), 粘稠状液体, 产率7%. 1H NMR (400 MHz, CDCl3) δ: 7.96 (d, J=8.8 Hz, 2H), 7.90 (s, 1H), 7.49 (d, J=8.8 Hz, 2H), 5.70 (dd, J=6.2, 7.6 Hz, 1H), 4.88 (dd, J=14.4, 6.2 Hz, 1H), 4.77 (dd, J=14.4, 7.6 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 189.0, 153.1, 141.1, 131.7, 130.5, 129.4, 50.5, 41.6; ESI-MS m/z: 415.1 [M+Na]+. Anal. calcd for C11H8Br2ClN3O: C 33.58, H 2.05, N 10.68; found C 33.86, H 2.25, N 10.79.
将第3.2.1节中的浓硫酸更换为冰醋酸, 其余的操作同上.
1-苯基-2-(1,2,4-三氮唑甲基)-2-溴-3-(1,2,4-三氮唑)-1-丙酮(Ⅱ'a): 白色固体, 产率72%. m.p. 122~123 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.32 (s, 2H), 8.11 (d, J=7.6 Hz, 2H), 7.96 (s, 2H), 7.61 (t, J=7.2 Hz, 1H), 7.50 (t, J=7.6 Hz, 2H), 5.07 (d, J=14.8 Hz, 2H), 4.90 (d, J=14.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 193.3, 152.5, 145.8, 134.9, 133.3, 129.8, 128.7, 60.7, 52.6; ESI- MS m/z: 361.2 [M+H]+, 383.3 [M+Na]+. Anal. calcd for C14H13BrN6O: C 46.55, H 3.63, N 23.27; found C 46.38, H 3.80, N 23.06.
1-(4-氟苯基)-2-(1,2,4-三氮唑甲基)-2-溴-3-(1,2,4-三氮唑)-1-丙酮(Ⅱ'b): 白色固体, 产率69%. m.p. 91~92 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.39 (s, 2H), 8.20 (m, 2H), 7.96 (s, 2H), 7.18 (t, J=8.4 Hz, 2H), 5.07 (d, J=15.0 Hz, 2H), 4.83 (d, J=15.0 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 191.7, 165.4, 152.6, 145.9, 132.7, 131.1, 115.9, 60.7, 52.6; ESI-MS m/z: 379.2 [M+H]+. Anal. calcd for C14H12BrFN6O: C 44.35, H 3.19, N 22.16; found C 44.54, H 3.33, N 22.09.
1-(4-氯苯基)-2-(1,2,4-三氮唑甲基)-2-溴-3-(1,2,4-三氮唑)-1-丙酮(Ⅱ'c): 白色固体, 产率74%. m.p. 125~126 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.40 (s, 2H), 8.11 (d, J=8.4 Hz, 2H), 7.96 (s, 2H), 7.48 (d, J=8.4 Hz, 2H), 5.06 (d, J=15.0 Hz, 2H), 4.80 (d, J=14.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 192.1, 152.6, 145.9, 139.7, 133.2, 131.4, 129.0, 60.7, 52.5; ESI-MS m/z: 395.1 [M+H]+. Anal. calcd for C14H12BrClN6O: C 42.50, H 3.06, N 21.24; found C 42.35, H 3.11, N 21.30.
1-(4-溴苯基)-2-(1,2,4-三氮唑甲基)-2-溴-3-(1,2,4-三氮唑)-1-丙酮(Ⅱ'd): 白色固体, 产率70%. m.p. 147~148 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.40 (s, 2H), 8.04 (d, J=8.6 Hz, 2H), 7.97 (s, 2H), 7.66 (d, J=8.6 Hz, 2H), 5.08 (d, J=14.8 Hz, 2H), 4.81 (d, J=14.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 192.4, 152.6, 145.8, 133.7, 132.0, 131.4, 128.4, 60.8, 52.5; ESI-MS m/z: 441.0 [M+H]+. Anal. calcd for C14H12Br2N6O: C 38.21, H 2.75, N 19.10; found C 38.08, H 2.79, N 19.25.
1-(4-甲基苯基)-2-(1,2,4-三氮唑甲基)-2-溴-3-(1,2,4-三氮唑)-1-丙酮(Ⅱ'e): 白色固体, 产率76%. m.p. 118~119 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.33 (s, 2H), 8.04 (d, J=8.0 Hz, 2H), 7.95 (s, 2H), 7.29 (d, J=8.0 Hz, 2H), 5.07 (d, J=14.8 Hz, 2H), 4.91 (d, J=15.0 Hz, 2H), 2.43 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 192.5, 152.4, 145.8, 132.0, 129.9, 129.7, 129.3, 60.6, 52.6, 21.7; ESI-MS m/z: 375.2 [M+H]+, 397.1 [M+Na]+. Anal. calcd for C15H15BrN6O: C 48.01, H 4.03, N 22.40; found C 47.87, H 4.07, N 22.27.
1-(4-甲氧基苯基)-2-(1,2,4-三氮唑甲基)-2-溴- 3-(1,2,4-三氮唑)-1-丙酮(Ⅱ'f): 白色固体, 产率65%. m.p. 139~140 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.35 (s, 2H), 8.18 (d, J=8.8 Hz, 2H), 7.96 (s, 2H), 6.98 (d, J=8.8 Hz, 2H), 5.07 (d, J=15.0 Hz, 2H), 4.93 (d, J=15.0 Hz, 2H), 3.90 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 191.0, 163.7, 152.5, 145.8, 132.4, 130.9, 127.0, 114.0, 60.7, 55.6, 52.9; ESI-MS m/z: 391.2 [M+H]+, 413.2 [M+Na]+. Anal. calcd for C15H15BrN6O2: C 46.05, H 3.86, N 21.48; found C 46.37, H 3.59, N 21.55.
在100 mL的三口瓶中依次加入1,2-二氯乙烷(40 mL)、Ⅱa~Ⅱf (17.9 mmol)、三乙胺(7.2 g, 71.4 mmol), 加热至40 ℃反应. TLC监控反应至Ⅱa~Ⅱf完全消失, 停止反应. 减压浓缩溶剂, 加适量蒸馏水搅拌3 h, 抽滤得到固体, 经乙醚和石油醚重结晶得到Ⅲa~Ⅲf.
1-苯基-3-(1,2,4-三氮唑)-2-丙烯-1-酮(Ⅲa): 白色固体, 产率82%. m.p. 168~169 ℃; 1H NMR (400MHz, CDCl3) δ: 8.46 (s, 1H), 8.19 (d, J=13.4 Hz, 1H), 8.15 (s, 1H), 8.08 (d, J=7.6 Hz, 2H), 7.80 (d, J=13.4 Hz, 1H), 7.66 (t, J=7.2 Hz, 1H), 7.56 (t, J=7.6 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 188.8, 153.7, 145.5, 137.2, 134.8, 133.7, 128.9, 128.6, 113.3; ESI-MS m/z: 200.1 [M+H]+. Anal. calcd for C11H9N3O: C 66.32, H 4.55, N 21.09; found C 66.45, H 4.52, N 21.13.
1-(4-氟苯基)-3-(1,2,4-三氮唑)-2-丙烯-1-酮(Ⅲb): 白色固体, 产率80%. m.p. 170~171 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.45 (s, 1H), 8.16 (d, J=13.4 Hz, 1H), 8.11 (s, 1H), 7.96 (d, J=7.6 Hz, 2H), 7.76 (d, J=13.4 Hz, 1H), 7.32 (d, J=7.6 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 187.1, 166.1, 153.7, 145.5, 134.9, 133.6, 131.3,, 116.1, 112.9; ESI-MS m/z: 218.2 [M+H]+. Anal. calcd for C11H8FN3O: C 60.83, H 3.71, N 19.35; found C 60.97, H 3.88, N 19.41.
1-(4-氯苯基)-3-(1,2,4-三氮唑)-2-丙烯-1-酮(Ⅲc): 白色固体, 产率75%. m.p. 176~177 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.42 (s, 1H), 8.15 (d, J=13.4 Hz, 1H), 8.12 (s, 1H), 8.00 (d, J=8.4 Hz, 2H), 7.73 (d, J=13.4 Hz, 1H), 7.51 (d, J=8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 187.5, 153.7, 145.6, 140.2, 135.5, 135.1, 130.0, 129.2, 112.8; ESI-MS m/z: 234.1 [M+H]+. Anal. calcd for C11H8ClN3O: C 56.55, H 3.45, N 17.98; found C 56.60, H 3.41, N 18.03.
1-(4-溴苯基)-3-(1,2,4-三氮唑)-2-丙烯-1-酮(Ⅲd): 白色固体, 产率78%. m.p. 190~191 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.47 (s, 1H), 8.19 (d, J=13.6 Hz, 1H), 8.16 (s, 1H), 7.96 (d, J=8.4 Hz, 2H), 7.76 (d, J=13.6 Hz, 1H), 7.71 (d, J=8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 187.7, 153.7, 135.9, 135.1, 132.2, 132.1, 130.0, 127.0, 112.7; ESI-MS m/z: 278.1 [M+H]+. Anal. calcd for C11H8BrN3O: C 47.51, H 2.90, N 15.11; found C 47.64, H 3.01, N 15.19.
1-(4-甲基苯基)-3-(1,2,4-三氮唑)-2-丙烯-1-酮(Ⅲe): 白色固体, 产率79%. m.p. 138~139 ℃; 1H NMR(400 MHz, CDCl3) δ: 8.43 (s, 1H), 8.15 (d, J=13.4 Hz, 1H), 8.11 (s, 1H), 7.96 (d, J=8.4 Hz, 2H), 7.77 (d, J=13.4 Hz, 1H), 7.33 (d, J=8.4 Hz, 2H), 2.45 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 188.3, 153.5, 144.7, 134.7, 129.5, 128.7, 113.3, 21.7; ESI-MS m/z: 214.2[M+H]+. Anal. calcd for C12H11N3O: C 67.59, H 5.20, N 19.71; found C 67.70, H 5.34, N 19.80.
1-(4-甲氧基苯基)-3-(1,2,4-三氮唑)-2-丙烯-1-酮(Ⅲf): 白色固体, 产率70%. m.p. 162~163 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.42 (s, 1H), 8.13 (s, 2H), 8.06 (d, J=8.8 Hz, 2H), 7.77 (d, J=13.2 Hz, 1H), 7.00 (d, J=8.8 Hz, 2H), 3.90 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 187.0, 164.1, 153.5, 145.4, 145.2, 134.2, 131.0, 130.2, 114.1, 114.0, 113.3, 55.6; ESI-MS m/z: 230.2 [M+H]+. Anal. calcd for C12H11N3O2: C 62.87, H 4.84, N 18.33; found C 62.95, H 4.90, N 18.43.
1-苯基-2-(1,2,4-三氮唑甲基)-3-(1,2,4-三氮唑)-2-丙烯-1-酮(Ⅲ'a): 白色固体, 产率89%. m.p. 141~142 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.56 (s, 1H), 8.31 (s, 1H), 8.19 (s, 1H), 7.88 (s, 1H), 7.72 (d, J=7.4 Hz, 2H), 7.61 (t, J=7.6 Hz, 2H), 7.49 (t, J=7.6 Hz, 2H), 5.91 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 194.9, 154.1, 152.1, 146.8, 136.9, 134.1, 133.1, 129.5, 128.8, 125.0, 44.6; ESI-MS m/z: 281.1 [M+H]+, 303.0 [M+Na]+. Anal. calcd for C14H12N6O: C 59.99, H 4.32, N 29.98; found C 60.11, H 4.50, N 29.67.
1-(4-氟苯基)-2-(1,2,4-三氮唑甲基)-3-(1,2,4-三氮唑)-2-丙烯-1-酮(Ⅲ'b): 黄色液体, 产率87%. 1H NMR (400 MHz, CDCl3) δ: 8.56 (s, 1H), 8.31 (s, 1H), 8.19 (s, 1H), 7.86 (s, 1H), 7.79~7.78 (m, 2H), 7.62, (s, 1H), 7.16 (t, J=8.4 Hz, 2H), 5.90 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 193.4, 165.7, 154.1, 152.1, 146.8, 144.2, 133.3, 133.0, 132.2, 125.0, 116.1, 44.8; ESI-MS m/z: 299.0 [M+H]+. Anal. calcd for C14H11FN6O: C 56.37, H 3.72, N 28.18; found C 56.49, H 3.51, N 28.40.
1-(4-氯苯基)-2-(1,2,4-三氮唑甲基)-3-(1,2,4-三氮唑)-2-丙烯-1-酮(Ⅲ'c): 白色固体, 产率87%. m.p. 124~125 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.54 (s, 1H), 8.30 (s, 1H), 8.20 (s, 1H), 7.87 (s, 1H), 7.68 (d, J=8.4 Hz, 2H), 7.59, (s, 1H), 7.46 (d, J=8.4 Hz, 2H), 5.90 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 193.7, 154.2, 152.2, 146.8, 144.2, 139.7, 135.1, 133.6, 131.0, 129.2, 124.9, 44.7; ESI-MS m/z: 315.1 [M+H]+. Anal. calcd for C14H11Cl- N6O: C 53.43, H 3.52, N 26.70; found C 53.23, H 3.66, N 26.62.
1-(4-溴苯基)-2-(1,2,4-三氮唑甲基)-3-(1,2,4-三氮唑)-2-丙烯-1-酮(Ⅲ'd): 白色固体, 产率86%. m.p. 146~147 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.56 (s, 1H), 8.32 (s, 1H), 8.22 (s, 1H), 7.89 (s, 1H),7.65~7.63 (m, 5H), 5.92 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 193.9, 154.2, 152.2, 146.8, 144.2, 135.6, 133.6, 132.2, 131.0, 128.3, 124.9, 44.6; ESI-MS m/z: 359.2 [M+H]+, 381.1 [M+Na]+. Anal. calcd for C14H11BrN6O: C 46.82, H 3.09, N 23.40; found C 46.03, H 3.31, N 23.57.
1-(4-甲基苯基)-2-(1,2,4-三氮唑甲基)-3-(1,2,4-三氮唑)-2-丙烯-1-酮(Ⅲ'e): 白色固体, 产率91%. m.p. 82~83 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.55 (s, 1H), 8.29 (s, 1H), 8.18 (s, 1H), 7.87 (s, 1H),7.63 (d, J=8.6 Hz, 3H), 7.28 (d, J=7.8 Hz, 2H), 5.89 (s, 2H), 2.43 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 194.5, 154.1, 152.1, 146.7, 144.2, 134.1, 133.4, 129.7, 129.5, 125.2, 44.8, 21.7; ESI- MS m/z: 295.2 [M+H]+, 317.2 [M+Na]+. Anal. calcd for C15H14N6O: C 61.21, H 4.79, N 28.55; found C 61.35, H 4.50, N 28.71.
1-(4-甲氧基苯基)-2-(1,2,4-三氮唑甲基)-3-(1,2,4-三氮唑)-2-丙烯-1-酮(Ⅲ'f): 白色固体, 产率85%. m.p. 125~126 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.54 (s, 1H), 8.28 (s, 1H), 8.19 (s, 1H), 7.87 (s, 1H),7.75 (d, J=8.8 Hz, 2H), 7.57 (s, 1H) 6.95 (d, J=8.8 Hz, 2H), 5.89 (s, 2H), 3.88 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 193.3, 163.8, 153.9, 152.1, 146.5, 144.2, 132.3, 132.1, 129.2, 125.4, 114.1, 55.6, 45.1; ESI-MS m/z: 311.0 [M+H]+, 333.1 [M+Na]+. Anal. calcd for C15H14N6O2: C 58.06, H 4.55, N 27.08; found C 57.91, H 4.84, N 27.37.
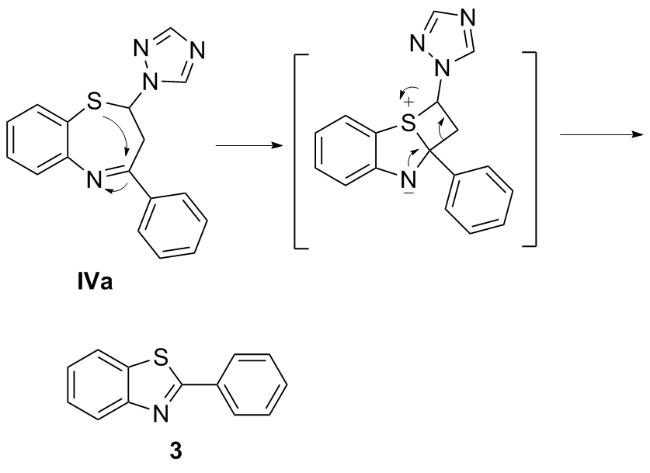
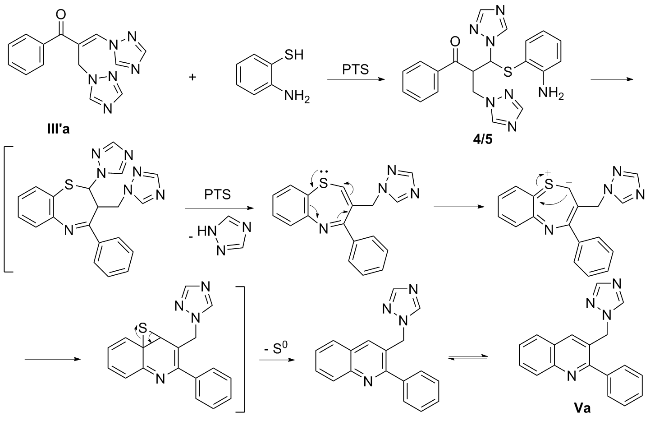
在50 mL的三口瓶中依次加入甲醇(15 mL)、Ⅲa~Ⅲf (6.0 mmol)、2-氨基苯硫酚(0.8 g, 6.0 mmol)和PTS (0.05 g, 2.4 mmol), 加热回流. TLC监控直至Ⅲa~Ⅲf消失, 停止反应. 减压浓缩甲醇, 剩余物中加入5 mL乙酸乙酯和3 mL石油醚, 冷冻, 析出固体, 过滤, 固体经乙醚重结晶得到Ⅳa~Ⅳf. Ⅲa反应的滤液经柱层析分离得到化合物3.
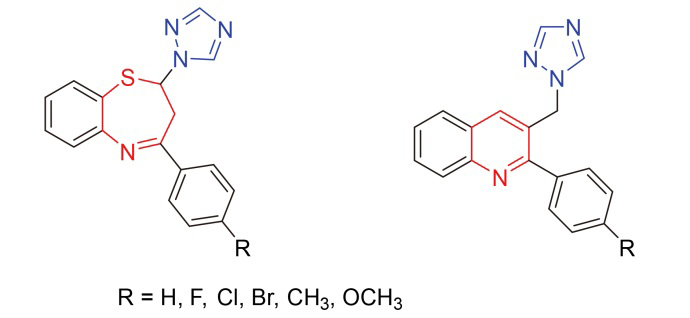
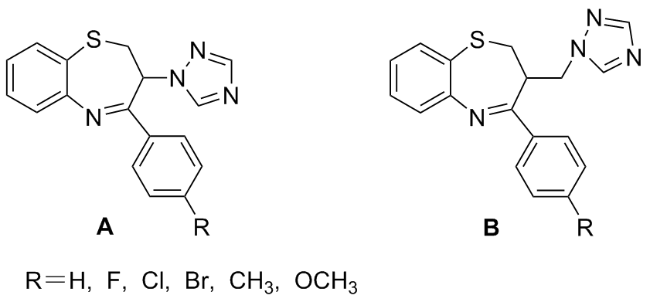
4-苯基-2-(1,2,4-三氮唑)-1,5-苯并硫氮杂䓬(Ⅳa): 白色固体, 产率73%. m.p. 107~108 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.40 (s, 1H), 8.10 (d, J=6.4Hz, 2H), 7.97 (s, 1H), 7.64 (d, J=6.4 Hz, 1H), 7.63~7.54 (m, 4H), 7.37 (d, J=6.8 Hz, 1H), 7.20 (t, J=7.0 Hz, 1H), 6.23(dd, J=12.2, 5.4 Hz, 1H), 3.78 (dd, J=12.8, 5.2 Hz, 1H), 3.13 (t,
J=12.6 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 165.7, 152.2, 151.8, 136.9, 131.7, 130.9, 129.0, 127.5, 119.6, 71.8, 36.4; ESI-MS m/z: 329.1 [M+Na]+. Anal. calcd for C17H14N4S: C 66.64, H 4.61, N 18.29; found C 66.78, H 4.60, N 18.23.
4-(4-氟苯基)-2-(1,2,4-三氮唑)-1,5-苯并硫氮杂䓬(Ⅳb): 白色固体, 产率76%. m.p. 82~83 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.41 (s, 1H), 8.10 (t, J=7.0 Hz, 2H), 7.97 (s, 1H), 7.64 (d, J=7.6 Hz, 1H), 7.54 (t, J=7.2 Hz, 1H), 7.35 (d, J=7.6 Hz, 1H), 7.20 (t, J=8.4 Hz, 3H), 6.21 (dd, J=12.4, 5.2 Hz, 1H), 3.74 (dd, J=13.0, 5.4 Hz, 1H), 3.12 (t, J=12.6 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 165.0, 164.5, 152.2, 151.7, 141.6, 135.0, 133.2, 131.0, 129.8, 125.9, 125.7, 119.6, 116.1, 71.7, 36.4; ESI-MS m/z: 363.2 [M+K]+. Anal. calcd for C17H13FN4S: C 62.95, H 4.04, N 17.27; found C 63.05, H 4.11, N 17.38.
4-(4-氯苯基)-2-(1,2,4-三氮唑)-1,5-苯并硫氮杂䓬(Ⅳc): 白色固体, 产率70%. m.p. 98~99 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.40 (s, 1H), 8.04 (d, J=6.0 Hz, 2H), 7.97 (s, 1H), 7.64 (d, J=6.8 Hz, 1H), 7.56~7.49 (m, 3H), 7.35 (d, J=6.8 Hz, 1H), 7.21 (t, J=7.6 Hz, 1H), 6.20 (dd, J=12.2, 5.4 Hz, 1H), 3.73 (dd, J=12.8, 5.2 Hz, 1H), 3.11 (t, J=12.6 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 164.5, 151.6, 138.0, 135.4, 135.0, 131.0, 129.3, 128.8, 126.0, 125.7, 119.6, 71.7, 36.3; ESI-MS m/z: 363.2 [M+Na]+. Anal. calcd for C17H13ClN4S: C 59.91, H 3.84, N 16.44; found C 60.11, H 3.98, N 16.51.
4-(4-溴苯基)-2-(1,2,4-三氮唑)-1,5-苯并硫氮杂䓬(Ⅳd): 白色固体, 产率68%. m.p. 103~104 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.40 (s, 1H), 7.96 (t, J=4.4 Hz, 3H), 7.64 (t, J=6.6 Hz, 3H), 7.54 (t, J=7.2 Hz, 1H), 7.35 (d, J=7.2 Hz, 1H), 7.20 (t, J=7.2 Hz, 1H), 6.20 (dd, J=12.4, 5.2 Hz, 1H), 3.71 (dd, J=12.8, 5.2 Hz, 1H), 3.11 (t, J=12.6 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 164.6, 152.3, 151.6, 141.5, 135.8, 135.0, 132.2, 131.0, 129.0, 126.5, 125.9, 125.7, 119.6, 71.7, 36.3; ESI-MS m/z: 385.2 [M+H]+. Anal. calcd for C17H13BrN4S: C 53.00, H 3.40, N 14.54; found C 53.12, H 3.49, N 14.64.
4-(4-甲基苯基)-2-(1,2,4-三氮唑)-1,5-苯并硫氮杂䓬(Ⅳe): 白色固体, 产率71%. m.p. 117~118 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.39 (s, 1H), 7.97 (t, J=7.2 Hz, 3H), 7.62 (d, J=6.4 Hz, 1H), 7.54 (t, J=7.0 Hz, 1H), 7.36~7.31 (m, 3H), 7.18 (t, J=6.8 Hz, 1H), 6.21 (dd, J=12.4, 5.2 Hz, 1H), 3.75 (dd, J=12.6, 5.4 Hz, 1H), 3.10 (t, J=12.6 Hz, 1H), 2.45 (s, 1H); 13C NMR (100 MHz, CDCl3) δ: 165.6, 152.2, 152.0, 142.3, 141.5, 134.9, 134.3, 130.9, 129.8, 127.5, 125.6, 119.7, 71.8, 36.4, 21.6; ESI-MS m/z: 321.4 [M+H]+. Anal. calcd for C18H16N4S: C 67.47, H 5.03, N 17.49; found C 67.60, H 4.99, N 17.65.
4-(4-甲氧基苯基)-2-(1,2,4-三氮唑)-1,5-苯并硫氮杂䓬(Ⅳf): 白色固体, 产率70%. m.p. 95~96 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.40 (s, 1H), 8.06 (t, J=8.8 Hz, 2H), 7.96 (s, 1H), 7.62 (d, J=7.6 Hz, 1H), 7.51 (t, J=7.0 Hz, 1H), 7.34 (d, J=6.8 Hz, 2H), 7.17 (t, J=6.8 Hz, 1H), 7.01 (d, J=8.8 Hz, 2H), 6.20 (dd, J=12.4, 5.2 Hz, 1H), 3.90 (s, 3H), 3.74 (dd, J=12.8, 5.2 Hz, 1H), 3.10 (t, J=12.6 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 164.9, 162.6, 152.1, 134.9, 130.9, 129.5, 129.3, 125.6, 125.4, 119.6, 114.3, 71.8, 55.5, 36.2; ESI-MS m/z: 337.1 [M+H]+. Anal. calcd for C18H16N4OS: C 64.27, H 4.79, N 16.65; found C 64.42, H 4.91, N 16.83.
2-苯基苯并噻唑(3): 白色固体, 产率16%. m.p. 93~94 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.11~8.15 (m, 3H), 7.95 (d, J=7.9 Hz, 1H), 7.57~7.51 (m, 4H), 7.43 (t, J=7.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 168.1, 154.1, 135.1, 133.6, 131.0 129.0, 127.6, 126.3, 125.2, 123.2, 121.6; ESI-MS m/z: 212.1 [M+H]+. Anal. calcd for C13H9NS: C 73.90, H 4.29, N 6.63; found C 73.59, H 4.32, N 6.70.
在50 mL的三口瓶中依次加入甲苯(15 mL)、Ⅲ'a~Ⅲ'f (10.0 mmol)、2-氨基苯硫酚(1.3 g, 10.0 mmol)和PTS (0.8 g, 4.0 mmol), 加热回流反应, TLC 监控至Ⅲ'a~Ⅲ'f消失, 停止反应, 加入适量三乙胺调节pH至中性, 减压浓缩溶剂, 剩余物经柱层析分离得到Ⅴa~Ⅴf.
2-苯基-3-(1,2,4-三氮唑甲基)喹啉(Ⅴa): 白色固体, 产率83%. m.p. 120~121 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.14 (d, J=8.4 Hz, 1H), 8.04 (s, 1H), 7.93 (s, 1H), 7.82 (d, J=8.0 Hz, 1H), 7.75 (t, J=7.0 Hz, 1H), 7.68 (s, 1H), 7.57 (t, J=7.4 Hz, 1H), 7.51~7.44 (m, 5H), 5.48 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 159.0, 152.2, 147.6, 143.5, 139.3, 137.2, 130.5, 129.4, 129.0, 128.9, 128.5, 127.6, 127.2, 127.0, 126.4, 51.3; ESI-MS m/z: 287.1 [M+H]+. Anal. calcd for C18H14N4: C 75.50, H 4.93, N 19.57; found C 75.72, H 5.00, N 19.56.
2-(4-氟苯基)-3-(1,2,4-三氮唑甲基)喹啉(Ⅴb): 白色固体, 产率80%. m.p. 108~109 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.14 (d, J=8.4 Hz, 1H), 8.04 (s, 1H), 7.97 (s, 1H), 7.85~7.83 (m, 2H), 7.78 (t, J=7.6 Hz, 1H), 7.60 (t,
J=7.0 Hz, 1H), 7.50~7.46 (m, 2H), 7.21 (t, J=8.6 Hz, 2H), 5.50 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 163.2, 157.9, 152.3, 147.5, 137.4, 135.3, 130.8, 130.5, 129.3, 127.6, 127.4, 127.1, 126.2, 116.0, 51.3; ESI-MS m/z: 305.1 [M+H]+. Anal. calcd for C18H13FN4: C 71.04, H 4.31, N 18.41; found C 71.27, H 4.35, N 18.56.
2-(4-氯苯基)-3-(1,2,4-三氮唑甲基)喹啉(Ⅴc): 白色固体, 产率82%. m.p. 133~134 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.13 (d, J=8.4 Hz, 1H), 8.03 (s, 1H), 7.98 (s, 1H), 7.87 (s, 1H), 7.83 (d, J=8.2 Hz, 1H), 7.77 (t, J=7.6 Hz, 1H), 7.59 (t, J=7.4 Hz, 1H), 7.50 (d, J=8.2 Hz, 1H), 7.44 (d, J=8.2 Hz, 1H), 5.49 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 157.7, 147.6, 137.7, 137.3, 135.3, 130.7, 130.0, 129.4, 129.1, 127.7, 127.5, 127.1, 126.1, 51.3; ESI-MS m/z: 321.0 [M+H]+. Anal. calcd for C18H13ClN4: C 67.40, H 4.09, N 17.47; found C 67.13, H 4.05, N 17.40.
2-(4-溴苯基)-3-(1,2,4-三氮唑甲基)喹啉(Ⅴd): 白色固体, 产率79%. m.p. 169~170 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.15 (d, J=8.4 Hz, 1H), 8.03 (s, 1H),7.97 (s, 1H), 7.84~7.82 (m, 2H), 7.77 (t, J=7.0 Hz, 1H), 7.66 (d, J=8.2 Hz, 2H), 7.60 (t, J=7.4 Hz, 1H), 7.38 (d, J=8.2 Hz, 1H), 5.49 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 157.7, 152.5, 147.5, 138.1, 137.4, 132.1, 130.8, 130.3, 129.4, 127.7, 127.5, 127.1, 126.2, 123.5, 51.1; ESI-MS m/z: 365.0 [M+H]+. Anal. calcd for C18H13BrN4: C 59.19, H 3.59, N 15.34; found C 59.43, H 3.45, N 15.43.
2-(4-甲基苯基)-3-(1,2,4-三氮唑甲基)喹啉(Ⅴe): 白色固体, 产率80%. m.p. 116~117 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.14 (d, J=8.4 Hz, 1H), 8.04 (s, 1H),7.95 (s, 1H), 7.82 (d, J=8.0 Hz, 1H), 7.77~7.73 (m, 2H), 7.57 (t, J=7.4 Hz, 1H), 7.36 (d, J=8.0 Hz, 2H), 7.32 (d, J=8.0 Hz, 2H), 5.51 (s, 2H), 2.45 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 159.1, 152.1, 147.7, 139.0, 137.2, 136.4, 130.5, 129.6, 129.4, 128.4, 127.6, 127.1, 127.0, 126.3, 51.4, 21.4; ESI-MS m/z: 301.2 [M+H]+. Anal. calcd for C19H16N4: C 75.98, H 5.37, N 18.65; found C 76.15, H 5.39, N 18.56.
2-(4-甲氧基苯基)-3-(1,2,4-三氮唑甲基)喹啉(Ⅴf): 白色固体, 产率82%. m.p. 115~116 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.14 (d, J=8.4 Hz, 1H), 8.02 (s, 1H),7.96 (s, 1H), 7.81 (d, J=8.0 Hz, 1H), 7.75~7.73 (m, 2H), 7.56 (t, J=7.2 Hz, 1H), 7.43 (d, J=8.6 Hz, 2H), 7.04 (d, J=8.6 Hz, 2H), 5.53 (s, 2H), 3.89 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 160.2, 158.8, 147.7, 137.1, 131.7, 130.5, 130.0, 129.4, 127.6, 127.1, 126.9, 126.5, 114.3, 55.5, 51.4; ESI-MS m/z: 317.1 [M+H]+. Anal. calcd for C19H16N4O: C 72.13, H 5.10, N 17.71; found C 71.89, H 5.15, N 17.66.
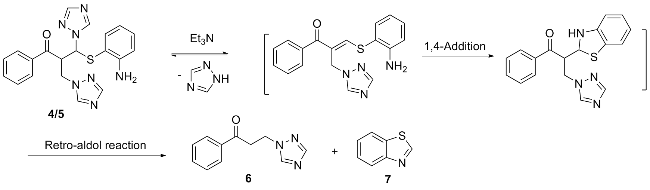
在50 mL的三口瓶中依次加入甲苯(15 mL)、Ⅲ'a (2.0 g, 7.1 mmol)和2-氨基苯硫酚(0.9 g, 7.1 mmol), 室温搅拌反应, TLC 监控至Ⅲ'a消失, 停止反应, 有固体析出, 抽滤, 经甲醇重结晶得到化合物4, 剩余物经柱层析分离得到化合物5.
1-苯基-2-(1,2,4-三氮唑甲基)-3-(2-氨基苯硫基)-3-(1,2,4-三氮唑)-1-丙酮(4): 白色固体, 产率49%. m.p. 179~180 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.90 (s, 1H), 7.86 (s, 1H), 7.76~7.58 (m, 3H), 7.56 (t, J=7.4 Hz, 1H), 7.46 (s, 1H), 7.40 (t, J=7.6 Hz, 2H), 7.17 (t, J=7.6 Hz, 1H), 6.96 (d, J=7.6 Hz, 1H), 6.70 (d, J=8.0 Hz, 1H), 6.59 (t, J=7.4 Hz, 1H), 5.87 (d, J=10.0 Hz, 1H), 5.26~5.21 (m, 1H), 5.00 (dd, J=14.0, 7.0 Hz, 1H), 4.91 (dd, J=14.2, 3.8 Hz, 1H), 4.41 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 197.3, 152.7, 152.6, 149.4, 144.0, 137.6, 135.3, 134.4, 132.3, 128.9, 128.4, 118.9, 115.4, 111.5, 65.0, 49.6, 48.8; ESI-MS m/z: 428.3 [M+Na]+. Anal. calcd for C20H19N7OS: C 59.24, H 4.72, N 24.18; found C 57.46, H 4.84, N 24.01.
5: 白色固体, 产率40%. m.p. 119~120 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.09 (s, 1H), 7.93 (d, J=7.6 Hz, 2H), 7.79 (s, 1H), 7.76 (s, 1H), 7.71 (s, 1H), 7.63 (t, J=7.2 Hz, 1H), 7.48 (t, J=7.6 Hz, 2H), 7.14 (t, 1H, J=7.4 Hz), 6.83 (d, 1H, J=7.4 Hz), 6.69 (d, 1H, J=8.0 Hz), 6.54 (t, 1H, J=7.4 Hz), 5.78 (d, 1H, J=10.0 Hz), 5.19~5.14 (m, 1H), 4.47 (dd, J=13.8, 7.8 Hz, 1H), 4.27 (dd, J=13.8, 3.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 197.6, 153.5, 152.4, 149.1, 144.6, 143.8, 137.3, 135.9, 134.6, 132.2, 129.1, 128.6, 118.7, 115.3, 112.2, 64.6, 49.2, 48.7; ESI-MS m/z: 428.3 [M+Na]+. Anal. calcd for C20H19- N7OS: C 59.24, H 4.72, N 24.18; found C 59.44, H 4.87, N 24.00.
在50 mL的三口瓶中依次加入甲苯(15 mL)、4 (1.2 g, 3.0 mmol)和三乙胺(0.2 mL, 1.5 mmol), 加热回流反应, TLC监控至4消失, 停止反应, 减压浓缩溶剂, 剩余物经柱层析分离得到化合物6和7.
1-苯基-3-(1,2,4,-三氮唑)-1-丙酮(6): 白色固体, 产率43%. m.p. 60~61 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.24 (s, 1H), 7.94~7.92 (m, 3H), 7.59 (t, J=7.2 Hz, 1H), 7.47 (t, J=7.6 Hz, 2H), 4.65 (t, J=6.4 Hz, 2H), 3.60 (t,
J=6.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 196.6, 136.0, 133.8, 128.8, 128.1, 44.1, 38.0; ESI-MS m/z: 202.1 [M+H]+, 224.2 [M+Na]+. Anal. calcd for C11H11N3O: C 65.66, H 5.51, N 20.88; found C 65.39, H 4.97, N 21.39.
苯并噻唑(7): 淡黄色液体, 产率40%. 1H NMR (400 MHz, CDCl3) δ: 9.00 (s, 1H), 8.16~7.44 (m, 4H); 13C NMR (100 MHz, CDCl3) δ: 152.9, 153.2, 133.7, 126.1, 125.5, 123.6, 121.9; ESI-MS m/z: 136.1 [M+H]+. Anal. calcd for C7H5NS: C 62.19, H 3.73, N 10.36; found C 61.94, H 3.94, N 10.23.