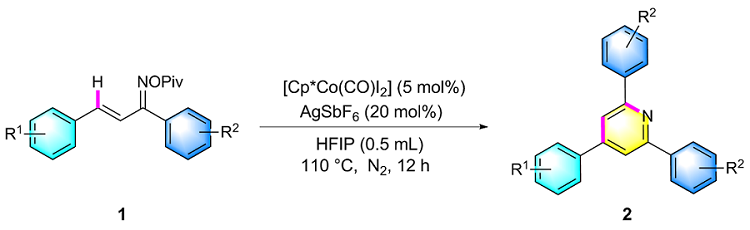
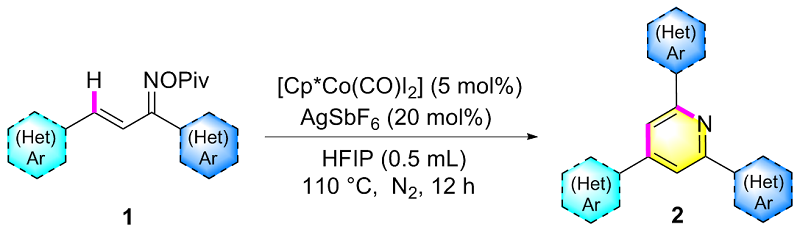
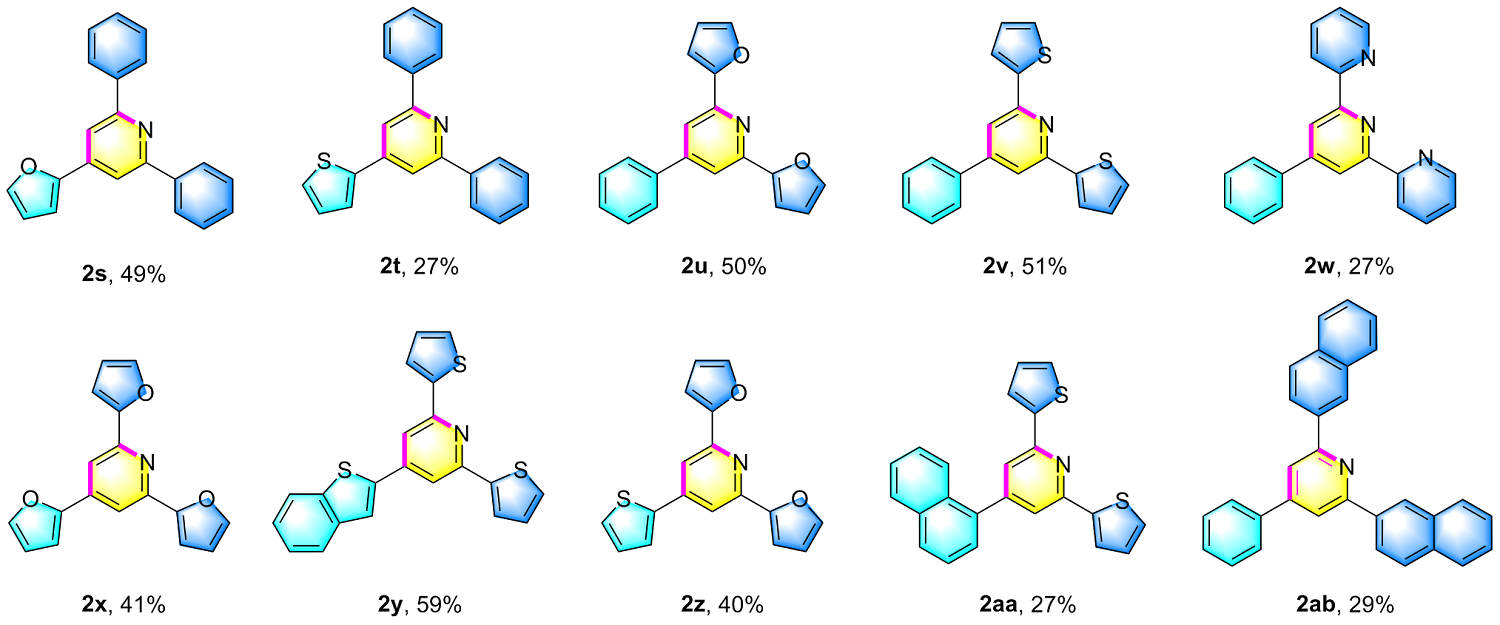
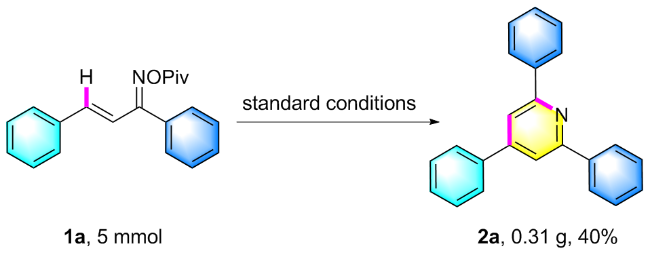
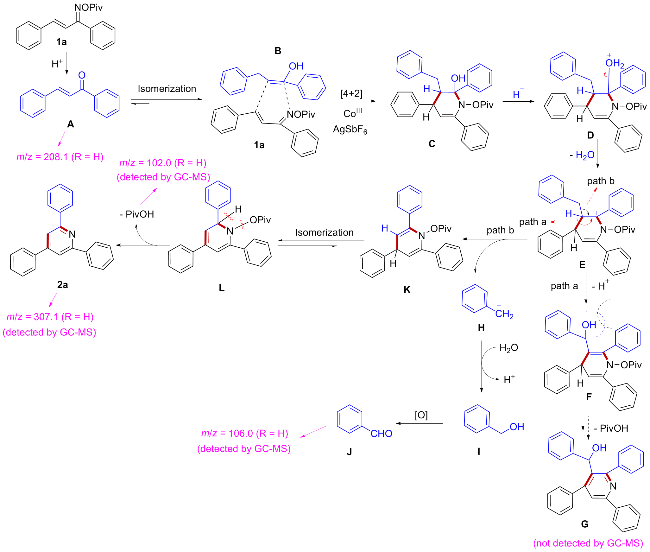
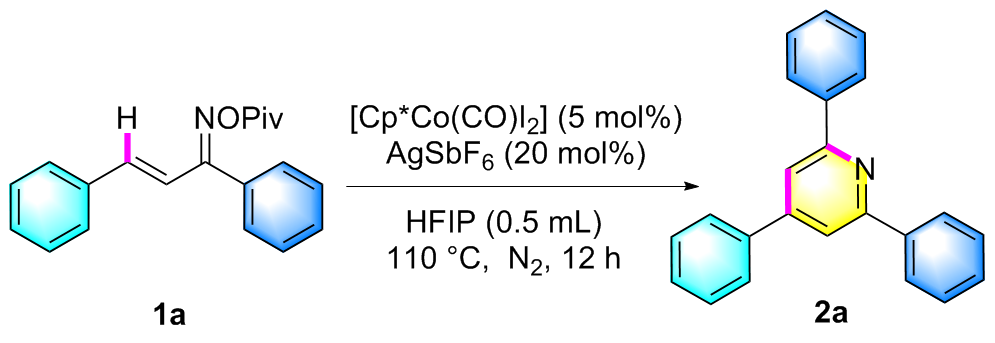在氮气保护条件下, 向25 mL密封反应管中依次加入磁力搅拌子、α,β-不饱和酮肟酯(1a, 61.5 mg, 0.2 mmol)、催化剂Cp*Co(CO)I₂ (4.8 mg, 0.01 mmol)、六氟锑酸银AgSbF₆ (13.7 mg, 20 mol%)以及溶剂六氟异丙醇HFIP (0.5 mL). 将反应体系置于110 ℃油浴中搅拌反应12 h. 待反应液冷却至室温后, 减压蒸除溶剂, 残留物经硅胶柱层析纯化(洗脱剂: PE/EA, V∶V=7∶1), 最终获得目标产物2a. 化合物2b~2z、2aa和2ab采用同样的方法制备得到.
2,4,6-三苯基吡啶(2a): 白色固体, 19.9 mg, 产率65%. m.p. 136.2~137.8 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.23 (d, J=7.4 Hz, 4H), 7.92 (s, 2H), 7.77 (d, J=7.3 Hz, 2H), 7.58~7.45 (m, 9H); 13C NMR (100 MHz, CDCl3) δ: 157.5, 150.2, 139.6, 139.1, 129.2, 129.1, 129.0, 128.7, 127.2, 127.1, 117.2; HRMS (ESI-TOF) calcd for C23H18N [M+H]+ 308.1434, found 308.1434.
2,6-二苯基-4-(对甲苯基)吡啶(2b): 白色固体, 20.2 mg, 产率63%. m.p. 108.8~109.7 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.22 (d, J=7.7 Hz, 4H), 7.90 (s, 2H), 7.68 (d, J=7.9 Hz, 2H), 7.53 (t, J=7.5 Hz, 4H), 7.46 (t, J=7.3 Hz, 2H), 7.36 (d, J=7.8 Hz, 2H), 2.46 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 157.5, 150.1, 139.7, 139.1, 136.1, 129.9, 129.0, 128.7, 127.2, 127.0, 116.9, 21.3; HRMS (ESI-TOF) calcd for C24H20N [M+H]+ 322.1590, found 322.1590.
4-(4-氯苯基)-2,6-二苯基吡啶(2c): 白色固体, 17.1 mg, 产率50%. m.p. 118.6~119.9 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.21 (d, J=7.7 Hz, 4H), 7.86 (s, 2H), 7.70 (d, J=7.9 Hz, 2H), 7.51 (ddd, J=23.3, 12.0, 7.3 Hz, 8H); 13C NMR (100 MHz, CDCl3) δ: 157.7, 149.0, 139.4, 137.5, 135.2, 129.4, 129.2, 128.8, 128.5, 127.1, 116.8; HRMS (ESI-TOF) calcd for C23H17ClN [M+H]+ 342.1044, found 342.1045.
4-(4-溴苯基)-2,6-二苯基吡啶(2d): 白色固体, 19.7 mg, 产率51%. m.p. 116.3~118.5 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.25~8.17 (m, 4H), 7.86 (d, J=1.6 Hz, 2H), 7.65 (td, J=8.5, 4.2 Hz, 4H), 7.54 (t, J=7.3 Hz, 4H), 7.47 (dd, J=7.6, 5.9 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 157.7, 149.0, 139.4, 138.0, 132.3, 129.2, 128.8, 127.2, 123.4, 116.8; HRMS (ESI-TOF) calcd for C23H17BrN [M+H]+ 386.0539, found 386.0541.
4-(4-碘苯基)-2,6-二苯基吡啶(2e): 白色固体, 19.5 mg, 产率45%. m.p. 120.7~121.9 ℃; 1H NMR (400 MHz, CDCl3) δ: 12.81 (s, 1H), 7.79 (s, 1H), 7.48~7.41 (m, 1H), 7.27~7.14 (m, 3H), 6.91 (s, 1H), 6.67 (s, 2H), 2.07 (s, 6H); 13C NMR (100 MHz, CDCl3) δ: 157.8, 149.1, 139.4, 138.6, 138.3, 129.2, 128.9, 128.8, 127.2, 116.7, 95.1; HRMS (ESI-TOF) calcd for C23H17IN [M+H]+ 434.0400, found 434.0399.
2,6-二苯基-4-间甲苯基吡啶(2f): 淡黄色油状物, 19.3 mg, 产率60%. 1H NMR (400 MHz, CDCl3) δ: 8.23 (dd, J=5.3, 3.3 Hz, 4H), 7.90 (s, 2H), 7.58~7.51 (m, 6H), 7.50~7.43 (m, 3H), 7.31 (d, J=7.7 Hz, 1H), 2.50 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 157.5, 150.4, 139.7, 139.1, 138.9, 129.7, 129.04, 129.03, 128.7, 127.9, 127.2, 124.3, 117.2, 21.6; HRMS (ESI-TOF) calcd for C24H20N [M+ H]+ 322.1590, found 322.1591.
4-(3-氯苯基)-2,6-二苯基吡啶(2g): 淡黄色油状物, 19.1 mg, 产率56%. 1H NMR (400 MHz, CDCl3) δ: 8.27~8.18 (m, 4H), 7.86 (s, 2H), 7.74 (s, 1H), 7.66~7.61 (m, 1H), 7.56~7.52 (m, 4H), 7.50~7.45 (m, 4H); 13C NMR (100 MHz, CDCl3) δ: 157.8, 148.9, 141.0, 139.4, 135.2, 130.4, 129.2, 129.0, 128.8, 127.4, 127.2, 125.4, 116.9; HRMS (ESI-TOF) calcd for C23H17ClN [M+H]+ 342.1044, found 342.1046.
4-(3-溴苯基)-2,6-二苯基吡啶(2h): 淡黄色油状物, 19.7 mg, 产率51%. 1H NMR (400 MHz, CDCl3) δ: 8.24~8.20 (m, 4H), 7.90 (t, J=1.8 Hz, 1H), 7.86 (s, 2H), 7.68 (d, J=7.8 Hz, 1H), 7.64~7.60 (m, 1H), 7.54 (dd, J=10.1, 4.7 Hz, 4H), 7.49~7.41 (m, 3H); 13C NMR (100 MHz, CDCl3) δ: 157.8, 148.8, 141.3, 139.4, 131.9, 130.7, 130.3, 129.2, 128.8, 127.2, 125.9, 123.3, 117.0; HRMS (ESI-TOF) calcd for C23H17BrN [M+H]+ 386.0539, found 386.0542.
2,6-二对甲苯基-4-苯基吡啶(2i): 白色固体, 22.1 mg, 产率63%. m.p. 118.7~120.2 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.12 (d, J=8.0 Hz, 4H), 7.86 (s, 2H), 7.76 (d, J=8.2 Hz, 2H), 7.58~7.47 (m, 3H), 7.34 (d, J=7.7 Hz, 4H), 2.45 (s, 6H); 13C NMR (100 MHz, CDCl3) δ: 157.4, 150.0, 139.3, 139.0, 136.9, 129.4, 129.1, 128.9, 127.2, 127.0, 116.5, 21.3; HRMS (ESI-TOF) calcd for C25H22N [M+H]+ 336.1747, found 336.1746.
2,6-双(4-甲氧基苯基)-4-苯基吡啶(2j): 淡黄色油状物, 22.4 mg, 产率61%. 1H NMR (400 MHz, CDCl3) δ: 8.17 (d, J=8.5 Hz, 4H), 7.79 (s, 2H), 7.75 (d, J=7.3 Hz, 2H), 7.56~7.47 (m, 3H), 7.05 (d, J=8.5 Hz, 4H), 3.90 (s, 6H); 13C NMR (100 MHz, CDCl3) δ: 160.5, 157.0, 150.0, 139.4, 132.4, 129.1, 128.9, 128.4, 127.2, 115.7, 114.1, 55.4; HRMS (ESI-TOF) calcd for C25H22NO2 [M+H]+ 368.1645, found 368.1645.
2,6-双(4-氟苯基)-4-苯基吡啶(2k): 白色固体, 18.9 mg, 产率55%. m.p. 164.0~165.8 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.19 (dd, J=8.6, 5.5 Hz, 4H), 7.84 (s, 2H), 7.75 (d, J=7.0 Hz, 2H), 7.53 (dt, J=13.9, 6.8 Hz, 3H), 7.21 (t, J=8.6 Hz, 4H); 13C NMR (100 MHz, CDCl3) δ: 164.9, 162.4, 156.5, 150.5, 138.9, 135.6, 129.2 (d, JC-F=4.4 Hz), 128.9 (d, JC-F=8.2 Hz), 127.2, 116.7, 115.6 (d, JC-F=21.5 Hz); 19F NMR (376 MHz, CDCl3) δ: 112.9; HRMS (ESI-TOF) calcd for C23H16F2N [M+H]+ 344.1245, found 344.1244.
2,6-双(4-氯苯基)-4-苯基吡啶(2l): 白色固体, 18.8 mg, 产率50%. m.p. 179.8~181.8 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.15 (d, J=8.4 Hz, 4H), 7.87 (s, 2H), 7.74 (d, J=7.9 Hz, 2H), 7.52 (dd, J=18.7, 8.0 Hz, 7H); 13C NMR (100 MHz, CDCl3) δ: 156.4, 150.6, 138.7, 137.8, 135.3, 129.2, 129.0, 128.4, 127.2, 117.1; HRMS (ESI- TOF) calcd for C23H16Cl2N [M+H]+ 376.0654, found 376.0653.
2,6-双(3-氟苯基)-4-苯基吡啶(2m): 白色固体, 18.9 mg, 产率55%. m.p. 120.4~122.1 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.98~7.94 (m, 4H), 7.91 (s, 2H), 7.77~7.74 (m, 2H), 7.57~7.46 (m, 5H), 7.19~7.14 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 164.6, 162.2, 156.3, 150.7, 141.7 (d, J=7.8 Hz), 138.7, 130.2 (d, J=8.2 Hz), 129.3 (d, J=1.6 Hz), 127.2, 122.6 (d, J=2.7 Hz), 117.7, 116.0 (d, J=21.2 Hz), 114.1 (d, J=23.0 Hz); 19F NMR (376 MHz, CDCl3) δ: 112.9; HRMS (ESI-TOF) calcd for C23H16F2N [M+H]+ 344.1245, found 344.1244.
2,6-双(3-氯苯基)-4-苯基吡啶(2n): 白色固体, 19.0 mg, 产率51%. m.p. 178.7~179.6 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.20 (d, J=1.8 Hz, 2H), 8.08 (dt, J=7.1, 1.7 Hz, 2H), 7.90 (s, 2H), 7.77~7.74 (m, 2H), 7.59~7.51 (m, 3H), 7.49~7.45 (m, 4H); 13C NMR (100 MHz, CDCl3) δ: 156.3, 150.8, 141.2, 134.9, 130.0, 129.3, 129.2, 127.3, 127.2, 125.3, 117.7; HRMS (ESI-TOF) calcd for C23H16Cl2N [M+H]+ 376.0654, found 376.0653.
2,6-双(3-溴苯基)-4-苯基吡啶(2o): 白色固体, 24.7 mg, 产率53%. m.p. 145.6~146.8 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.34 (t, J=1.8 Hz, 2H), 8.15~8.11 (m, 2H), 7.89 (s, 2H), 7.77~7.73 (m, 2H), 7.61~7.51 (m, 5H), 7.41 (t, J=7.9 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 156.2, 141.5, 132.1, 130.3, 130.2, 129.3, 129.3, 127.2, 125.8, 123.1, 117.8; HRMS (ESI-TOF) calcd for C23H16- Br2N [M+H]+ 463.9644, found 463.9641.
2,4,6-三对甲苯基吡啶(2p): 白色固体, 19.9 mg, 产率57%. m.p. 174.9~176.2 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.11 (d, J=8.1 Hz, 4H), 7.84 (s, 2H), 7.66 (d, J=8.0 Hz, 2H), 7.34 (t, J=7.8 Hz, 6H), 2.46 (s, 3H), 2.45 (s, 6H); 13C NMR (100 MHz, CDCl3) δ: 157.4, 149.9, 139.0, 138.9, 137.0, 136.3, 129.8, 129.4, 127.0, 116.3, 21.3, 21.2; HRMS (ESI-TOF) calcd for C26H24N [M+H]+ 350.1903, found 350.1901.
2,6-双(4-甲氧基苯基)-4-对甲苯基吡啶(2q): 淡黄色油状物, 19.5 mg, 产率51%. 1H NMR (400 MHz, CDCl3) δ: 8.17 (d, J=8.4 Hz, 4H), 7.77 (s, 2H), 7.65 (d, J=7.8 Hz, 2H), 7.34 (d, J=7.7 Hz, 2H), 7.04 (d, J=8.4 Hz, 4H), 3.90 (s, 6H), 2.45 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 160.5, 157.0, 149.9, 138.9, 136.4, 132.5, 129.8, 128.4, 127.0, 115.5, 114.1, 55.4, 21.3; HRMS (ESI-TOF) calcd for C26H24NO2 [M+H]+ 382.1802, found 382.1804.
2,4,6-三(4-氯苯基)吡啶(2r): 白色固体, 17.7 mg, 产率43%. m.p. 268.8~271.6 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.16~8.10 (m, 4H), 7.82 (s, 2H), 7.70~7.65 (m, 2H), 7.51 (t, J=9.0 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ: 156.6, 137.6, 135.5, 129.5, 129.4, 129.0, 128.9, 128.5, 128.4, 127.9, 116.8; HRMS (ESI-TOF) calcd for C23H15Cl3N [M+H]+ 410.0265, found 410.0262.
4-呋喃-2-基-2,6-二苯基吡啶(2s): 淡黄色固体, 14.5 mg, 产率49%. m.p. 220.3~221.9 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.24~8.18 (m, 4H), 7.95 (s, 2H), 7.61 (d, J=1.5 Hz, 1H), 7.53 (dd, J=10.1, 4.7 Hz, 4H), 7.49~7.44 (m, 2H), 7.00 (d, J=3.4 Hz, 1H), 6.59 (dd, J=3.4, 1.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 157.6, 152.1, 143.6, 139.5, 139.1, 129.1, 128.7, 127.1, 113.1, 112.1, 108.5; HRMS (ESI-TOF) calcd for C21H16NO [M+H]+ 298.1226, found 298.1226.
2,6-二苯基-4-噻吩-2-基吡啶(2t): 白色固体, 8.5 mg, 产率27%. m.p. 137.8~139.5 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.23~8.17 (m, 4H), 7.89 (s, 2H), 7.64 (d, J=2.9 Hz, 1H), 7.53 (t, J=7.4 Hz, 4H), 7.49~7.44 (m, 3H), 7.20 (dd, J=5.0, 3.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 157.8, 147.0, 143.1, 139.5, 129.2, 128.7, 128.4, 127.2, 126.9, 125.3, 115.4; HRMS (ESI-TOF) calcd for C21H16NS [M+H]+ 314.0998, found 314.0999.
2,6-二呋喃-2-基-4-苯基吡啶(2u): 淡黄色油状物, 14.4 mg, 产率50%. 1H NMR (400 MHz, CDCl3) δ: 7.82 (s, 2H), 7.79~7.75 (m, 2H), 7.58~7.47 (m, 5H), 7.21 (d, J=3.3 Hz, 2H), 6.58 (dd, J=3.4, 1.7 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 153.9, 149.9, 149.8, 143.3, 138.5, 129.2, 129.1, 127.1, 114.9, 112.1, 109.1; HRMS (ESI- TOF) calcd for C19H14NO2 [M+H]+ 288.1019, found 288.1019.
4-苯基-2,6-二噻吩-2-基吡啶(2v): 淡黄色油状物, 16.3 mg, 产率51%. 1H NMR (400 MHz, CDCl3) δ: 7.75~7.71 (m, 4H), 7.70 (s, 2H), 7.56~7.52 (m, 2H), 7.50 (d, J=7.1 Hz, 1H), 7.44 (d, J=5.1 Hz, 2H), 7.15 (dd, J=4.9, 3.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 152.7, 150.2, 144.9, 138.6, 129.1, 128.0, 127.8, 127.1, 124.9, 115.1; HRMS (ESI-TOF) calcd for C19H14NS2 [M+H]+ 320.0562, found 320.0563.
4'-苯基-2,2':6',2''-三联吡啶(2w): 淡褐色固体, 8.3 mg, 产率27%. m.p. 214.7~216.8 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.83~8.71 (m, 5H), 8.40 (dt, J=4.5, 2.5 Hz, 2H), 7.94 (td, J=7.7, 1.8 Hz, 1H), 7.61~7.52 (m, 6H), 7.46 (ddd, J=7.5, 4.7, 1.1 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 165.2, 149.5, 137.1, 130.9, 130.7, 128.9, 128.51, 128.47, 127.5, 125.3, 122.0, 110.7; HRMS (ESI-TOF) calcd for C21H16N3 [M+H]+ 310.1339, found 310.1338.
2,4,6-三呋喃-2-基吡啶(2x): 淡黄色固体, 11.3 mg, 产率41%. m.p. 99.1~100.6 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.82 (s, 2H), 7.58 (dd, J=8.5, 1.1 Hz, 3H), 7.19 (d, J=3.3 Hz, 2H), 6.99 (d, J=3.4 Hz, 1H), 6.57 (dt, J=3.5, 1.9 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 153.8, 151.7, 149.8, 143.8, 143.3, 138.8, 112.13, 112.06, 111.0, 109.2, 108.9; HRMS (ESI-TOF) calcd for C17H12NO3 [M+H]+ 278.0812, found 278.0810.
4-苯并[b]噻吩-2-基-2,6-二噻吩-2-基吡啶(2y): 淡黄色油状物, 22.0 mg, 产率59%. 1H NMR (400 MHz, CDCl3) δ: 7.91~7.86 (m, 2H), 7.84 (s, 1H), 7.77~7.74 (m, 4H), 7.46 (d, J=5.0 Hz, 2H), 7.44~7.40 (m, 2H), 7.17 (dd, J=4.8, 3.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 153.0, 144.6, 143.1, 141.2, 140.2, 139.9, 128.1, 128.0, 125.51, 125.11, 125.0, 124.3, 122.5, 122.2, 113.8; HRMS (ESI-TOF) calcd for C21H14NS3 [M+H]+ 376.0283, found 376.0281.
2,6-二呋喃-2-基-4-噻吩-2-基吡啶(2z): 淡黄色油状物, 11.7 mg, 产率40%. 1H NMR (400 MHz, CDCl3) δ: 7.78 (s, 2H), 7.64 (dd, J=3.7, 1.0 Hz, 1H), 7.58 (d, J=0.8 Hz, 2H), 7.45 (dd, J=5.0, 0.9 Hz, 1H), 7.20~7.16 (m, 3H), 6.57 (dd, J=3.4, 1.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 153.7, 149.9, 143.4, 142.8, 141.6, 128.4, 127.1, 125.5, 113.0, 112.1, 109.3; HRMS (ESI-TOF) calcd for C17H12NO2S [M+H]+ 294.0583, found 294.0583.
4-(萘-1-基)-2,6-二噻吩-2-基吡啶(2aa): 淡黄色油状物, 10.0 mg, 产率27%. 1H NMR (400 MHz, CDCl3) δ: 7.94 (dd, J=16.2, 8.3 Hz, 3H), 7.67 (dd, J=3.7, 1.1 Hz, 2H), 7.64 (s, 2H), 7.63~7.55 (m, 2H), 7.55~7.50 (m, 2H), 7.44 (dd, J=5.0, 1.1 Hz, 2H), 7.13 (dd, J=5.0, 3.7 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 152.3, 150.3, 144.9, 137.6, 133.9, 131.0, 128.9, 128.6, 128.00, 127.96, 126.8, 126.6, 126.3, 125.40, 125.36, 125.0, 118.2; HRMS (ESI-TOF) calcd for C23H16NS2 [M+H]+ 370.0719, found 370.0716.
2,6-二萘-2-基-4-苯基吡啶(2ab): 淡黄色油状物, 12.0 mg, 产率29%. 1H NMR (400 MHz, CDCl3) δ: 8.72 (d, J=1.0 Hz, 2H), 8.44 (dd, J=8.6, 1.8 Hz, 2H), 8.09 (s, 2H), 8.03 (dd, J=9.0, 2.6 Hz, 4H), 7.95~7.91 (m, 2H), 7.87~7.83 (m, 2H), 7.61~7.52 (m, 7H); 13C NMR (100 MHz, CDCl3) δ: 157.6, 150.4, 139.2, 137.1, 133.9, 133.6, 129.2, 129.1, 128.8, 128.48, 128.45, 127.8, 127.3, 126.6, 126.3, 125.0, 117.5; HRMS (ESI-TOF) calcd for C31H22N [M+H]+ 408.1747, found 408.1750.