中间体2-氯苯甲酰乙酸甲酯(
2)依照文献[
15]合成, 收率83%.
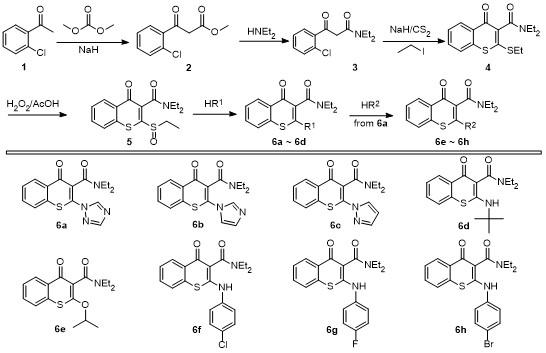
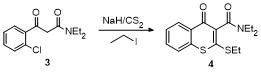
将合成的中间体2 (2.12 g, 10 mmol)与二乙胺(0.146 g, 20 mmol)投入到盛有20 mL甲苯的三口反应瓶中, 加热至90 ℃反应10 h. 反应溶液冷却至室温后, 用水(10 mL×2)洗涤, 将洗涤后的有机相用无水硫酸钠干燥, 真空浓缩, 残余物用硅胶柱分离(石油醚/乙酸乙酯, V∶ V=5∶1), 得2.18 g (8.61 mmol)化合物3, 淡黄色液体, 收率86%. 该化合物在测试条件下同时呈现出烯醇式和二酮式结构, 1H NMR和13C NMR能够清晰地表明烯醇式和二酮式结构比例大约为4∶1. 1H NMR (400 Hz, CDCl3) δ: 15.44 (s, 1H), 7.68~7.29 (m, 5H), 5.66 (s, 1H), 3.48~3.32 (m, 5H), 1.23~1.10 (m, 7H); 13C NMR (100 Hz, CDCl3) δ: 196.59, 171.02, 169.96, 165.67, 138.30, 135.03, 132.20, 131.83, 130.98, 130.51, 130.45, 130.16, 130.08, 127.06, 126.81, 90.89, 48.71, 42.75, 42.33, 40.57, 40.15, 14.40, 14.23, 13.32, 12.85; HRMS (ESI) calcd for C13H17ClNO2 [M+H]+ 254.0948, found 254.0941.
将化合物3 (2.53 g, 10 mmol)投入盛有15 mL DMSO的三口反应瓶中, 然后投入60% NaH (0.8 g, 20 mmol), 搅拌下升温至40 ℃. 然后将含有CS2 (1.14 g, 15 mmol)的DMSO (4 mL)溶液缓慢滴入反应瓶中(40 ℃). 反应完毕后, 冷却至室温, 边搅拌边滴加碘乙烷(2.34 g, 15 mmol)的DMSO (5 mL)溶液, 滴毕, 室温反应至反应完全. 将反应液倒入50 mL水中, 调节pH值至中性, 然后用乙酸乙酯(30 mL)萃取, 有机相水洗(15 mL×2), 干燥后浓缩, 残余物用硅胶柱分离(石油 醚/乙酸乙酯, V∶V=10∶1), 得1.82 g (5.67 mmol)化合物4, 淡黄色液体, 收率57%. 1H NMR (400 Hz, CDCl3) δ: 8.44 (d, J=8 Hz, 1H), 7.60~7.28 (m, 3H), 3.70~3.63 (m, 1H), 3.55~3.48 (m, 1H), 3.24~3.10 (m, 4H), 1.41 (t, J=7.4 Hz, 3H), 1.28 (t, J=7 Hz, 3H), 1.13 (t, J=7 Hz, 3H); 13C NMR (100 Hz, CDCl3) δ: 175.66, 164.98, 149.59, 136.98, 133.34, 131.59, 130.34, 129.13, 127.97, 125.33, 42.85, 39.11, 27.95, 14.55, 14.15, 12.65.
将化合物5 (337 mg, 1 mmol)和各种唑杂环化合物(2 mmol)加入到盛有5 mL DMF的50 mL三口瓶中, 110 ℃加热12 h. 反应混合物冷却至室温后, 加入30 mL二氯甲烷稀释, 用水(20 mL×2)洗涤, 将洗涤后的有机相用无水硫酸钠干燥, 真空浓缩, 用薄层色谱分离(石油醚/乙酸乙酯, V∶V=1∶1), 得到目标化合物6a~6d.
N,N-二乙基-4-氧-2-(1H-1,2,4-三唑-1-基)-4H-硫色酮-3-甲酰胺(6a): 淡黄色粉末(纯度98%), 收率67%. m.p. 130.6~133.0 ℃; 1H NMR (400 Hz, CDCl3) δ: 8.92 (s, 1H), 8.55 (d, J=7.6 Hz, 1H), 8.17 (s, 1H), 7.74~7.63 (m, 3H), 3.57~3.52 (m, 2H), 3.20~3.15 (m, 2H), 1.21 (dq, J=2.7, 7 Hz), 0.90 (dq, J=2.5, 7 Hz); 13C NMR (100 Hz, CDCl3) δ: 178.50, 163.88, 153.34, 145.06, 142.73, 134.78, 132.91, 129.71, 129.30, 128.59, 127.21, 126.54, 43.29, 39.76, 13.79, 12.48; HRMS (ESI) calcd for C16H17N4O2S [M+H]+ 329.1072, found 329.1064.
N,N-二乙基-2-(1H-咪唑-1-基)-4-氧代-4H-硫色酮 基-3-甲酰胺(6b): 白色粉末(纯度97%), 收率42%. m.p. 127.5~129.0 ℃; 1H NMR (400 Hz, CDCl3) δ: 8.54 (dd, J=1, 8.2 Hz), 8.00 (s, 1H), 7.75~7.71 (m, 1H), 7.65~7.60 (m, 2H), 7.44 (t, J=1.4 Hz, 1H), 7.21 (s, 1H), 3.64~3.58 (m, 1H), 3.36~3.31 (m, 1H), 3.16~3.05 (m, 2H), 1.12 (t, J=7.2 Hz, 3H), 0.91 (t, J=7.2 Hz, 3H); 13C NMR (100 Hz, CDCl3) δ: 178.52, 163.42, 142.12, 137.16, 134.14, 132.86, 131.10, 129.91, 129.51, 129.44, 128.72, 126.26, 120.01, 43.01, 39.28, 13.84, 12.38; HRMS (ESI) calcd for C17H18N3O2S [M+H]+ 328.1120, found 328.1111.
N,N-二乙基-4-氧-2-(1H-吡唑-1-基)-4H-硫色酮-3-甲酰胺(6c): 白色粉末(纯度99%), 收率46%. m.p. 127.4~129.3 ℃; 1H NMR (400 Hz, CDCl3) δ: 8.52 (dd, J=8.2, 1 Hz, 1H), 8.32 (d, J=2.4 Hz, 1H), 7.82~7.56 (m, 4H), 6.51 (q, J=2.4 Hz, 1H), 3.64~3.48 (m, 2H), 3.15 (q, J=7.2 Hz, 2H), 1.23 (t, J=7.2 Hz, 3H), 0.84 (t, J=7.2 Hz, 3H); 13C NMR (100 Hz, CDCl3) δ: 178.94, 164.85, 146.54, 143.30, 135.36, 132.47, 131.75, 129.58, 128.99, 128.04, 126.35, 124.06, 109.58, 43.18, 39.57, 13.52, 12.42; HRMS (ESI) calcd for C17H18N3O2S [M+H]+ 328.1120, found 328.1112.
2-(叔丁基氨基)-N,N-二乙基-4-氧代-4H-硫色酮基- 3-甲酰胺(6d): 红色粉末(纯度96%), 收率53%. m.p. 132.6~134.7 ℃; 1H NMR (400 Hz, CDCl3) δ: 8.43~8.41 (m, 1H), 7.52~7.29 (m, 3H), 6.34 (s, 1H), 3.59~3.54 (m, 2H), 3.36~3.13 (m, 2H), 1.49 (s, 9H), 1.26 (t, J=7.2 Hz, 3H), 1.07 (t, J=7.2 Hz, 3H); 13C NMR (100 Hz, CDCl3) δ: 175.28, 167.58, 157.06, 131.91, 130.76, 130.35, 128.38, 127.19, 125.38, 109.83, 54.07, 43.39, 39.82, 29.90, 14.56, 13.07; HRMS (ESI) calcd for C18H25- N2O2S [M+H]+ 333.1637, found 333.1632.
将化合物6a (328 mg, 1 mmol)和异丙醇(120 mg, 2 mmol)加入到装有5 mL DMF的50 mL三口瓶中, 110 ℃加热12 h. 反应混合物冷却至室温后, 加入30 mL二氯甲烷稀释, 用水(20 mL×2)洗涤, 将洗涤后的有机相用无水硫酸钠干燥, 真空浓缩, 用薄层色谱分离(石油醚/乙酸乙酯, V∶V=1∶1), 得到131 mg (0.41 mmol) N,N-二乙基-2-异丙氧基-4-氧代-4H-硫色酮基-3-甲酰胺(6e), 淡黄色固体(纯度97%), 收率41%. m.p. 42.3~44.2 ℃; 1H NMR (400 Hz, CDCl3) δ: 8.50~8.48 (m, 1H), 7.60~7.49 (m, 3H), 4.90~4.87 (m, 1H), 3.76~3.71 (m, 1H), 3.46~3.41 (m, 1H), 3.27~3.22 (m, 2H), 1.46~1.44 (m, 6H), 1.26 (t, J=7 Hz, 3H), 1.12 (t, J=7 Hz, 3H); 13C NMR (400 Hz, CDCl3) δ: 178.75, 164.51, 164.34, 132.11, 131.59, 130.37, 128.91, 127.63, 126.34, 119.92, 76.62, 42.89, 39.05, 22.61, 22.36, 14.24, 12.72; HRMS (ESI) calcd for C17H22NO3S [M+H]+ 320.1320, found 320.1314.
将化合物6a (328 mg, 1 mmol)和取代苯胺(2 mmol)加入100 mL的封管反应器中, 加入5 mL二甲苯, 在密闭状态下165 ℃加热12 h. 反应混合物冷却至室温, 加入10 mL乙酸乙酯稀释, 用水(20 mL×2)洗涤, 将洗涤后的有机相用无水硫酸钠干燥, 真空浓缩, 用薄层色谱分离(石油醚/乙酸乙酯, V∶V=1∶1), 得到目标化合物6f~6h.
2-[(4-氯苯基)氨基]-N,N-二乙基-4-氧代-4H-硫色酮基-3-甲酰胺(6f): 白色粉末(纯度98%), 收率87%. m.p. 144.5~146.4 ℃; 1H NMR (400 Hz, CDCl3) δ: 8.46 (d, J=7.6 Hz, 1H), 8.36 (d, J=6.4 Hz, 1H), 7.56~7.47 (m, 2H), 7.42~7.37 (m, 3H), 7.30~7.27 (m, 2H), 3.87~3.82 (m, 1H), 3.46~3.27 (m, 3H), 1.32 (t, J=7.2 Hz, 3H), 1.18 (t, J=7.2 Hz, 3H); 13C NMR (100 Hz, CDCl3) δ: 176.39, 166.98, 156.87, 136.00, 132.48, 131.69, 131.29, 130.20, 129.79, 128.57, 127.49, 126.35, 125.83, 111.67, 43.33, 39.70, 14.50, 12.98; HRMS (ESI) calcd for C20H20Cl- N2O2S [M+H]+ 387.0934, Found 387.0926.
2-[(4-氟苯基)氨基]-N,N-二乙基-4-氧代-4H-硫色酮基-3-甲酰胺(6g): 淡黄色粉末(纯度98%), 收率78%. m.p. 71.1~71.8 ℃; 1H NMR (400 Hz, CDCl3) δ: 8.44 (d, J=7.6 Hz, 1H), 8.29 (s, 1H), 7.50~7.46 (m, 2H), 7.35~7.27 (m, 3H), 7.13~7.09 (m, 2H), 3.86~3.79 (m, 1H), 3.43~3.25 (m, 3H), 1.31 (t, J=7.2 Hz, 3H), 1.17 (t, J=7.2 Hz, 3H); 13C NMR (100 Hz, CDCl3) δ: 176.23, 167.05, 161.39 (d, J=246.3 Hz), 157.90, 133.26 (d, J=2.9 Hz), 131.71, 131.15, 130.21, 128.56, 127.79 (d, J=8.6 Hz), 127.39, 125.78, 116.59 (d, J=22.6 Hz), 110.85, 43.32, 39.70, 14.53, 13.00; HRMS (ESI) calcd for C20H20FN2O2S [M+H]+ 371.1230, found 371.1222.
2-[(4-溴苯基)氨基]-N,N-二乙基-4-氧代-4H-硫色酮基-3-甲酰胺(6h): 淡黄色粉末(纯度97%), 收率81%. m.p. 146.1~148.7 ℃; 1H NMR (400 Hz, CDCl3) δ: 8.44 (dd, J=7.8, 1.4 Hz, 1H), 8.35 (s, 1H), 7.54~7.45 (m, 4H), 7.37~7.35 (m, 1H), 7.21~7.19 (m, 2H), 3.84~3.79 (m, 1H), 3.43~3.26 (m, 3H), 1.30 (t, J=7.2 Hz, 3H), 1.16 (t, J=7.2 Hz, 3H); 13C NMR (100 Hz, CDCl3) δ: 176.41, 166.99, 156.60, 136.60, 132.74, 131.71, 131.29, 130.21, 128.58, 127.49, 126.50, 125.83, 120.17, 111.87, 43.35, 39.72, 14.48, 12.96; HRMS (ESI) calcd for C20H20Br- N2O2S [M+H]+ 431.0429, found 431.0424.
中间体
8和
9参考文献[
18]合成, 收率分别为98%和72%.
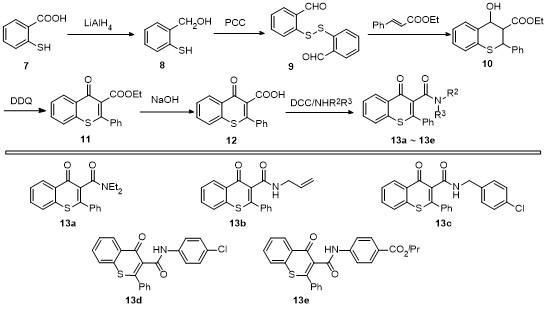
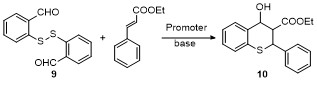
2-巯基苯甲醛二聚体(9): 褐色固体. m.p. 140.1~142.8 ℃; 1H NMR (400 Hz, CDCl3) δ: 10.23 (s, 2H), 7.88~7.77 (m, 4H), 7.52~7.37 (m, 4H); 13C NMR (100 Hz, CDCl3) δ: 191.83, 140.07, 134.68, 134.29, 133.85, 126.74, 126.33.
将肉桂酸乙酯(2.64 g, 0.015 mol)、2-巯基苯甲醛二聚体(9, 5.48 g, 0.02 mol)和三苯基膦(6.55 g, 0.025 mol)投入到盛有50 mL甲苯的三口反应瓶中, 搅拌, 升温至50 ℃后, 滴加四甲基胍(1.725 g, 0.015 mol), 7 h后降温, 将反应溶液倒入100 mL水中, 并用乙酸乙酯(30 mL×2)萃取, 有机相干燥浓缩后, 硅胶柱分离(石油醚/乙酸乙酯, V∶V=10∶1)得到3.44 g (0.011 mol) 4-羟基-2-苯基硫代色烷-3-甲酸乙酯(10), 淡黄色粉末, 收率73%. m.p. 96.7~98.2 ℃; 1H NMR (400 Hz, DMSO-d6) δ: 7.49~7.33 (m, 6H), 7.27~7.12 (m, 3H), 5.87 (d, J=6.0 Hz, 1H), 5.05 (dd, J=5.6 Hz, 1H), 4.92 (d, J=12.0 Hz, 1H), 4.04~3.88 (m, 2H), 3.47 (dd, J=12.0 Hz, 1H), 1.01 (t, J=7.2 Hz, 3H); 13C NMR (100 Hz, DMSO-d6) δ: 170.92, 140.26, 135.17, 133.50, 131.54, 128.89, 128.83, 128.79, 128.05, 124.99, 124.63, 68.93, 60.42, 50.80, 40.52, 14.26; MS(ESI) calcd for C18H18NaO3S [M+Na]+ 337.0874, found 337.0870.
将化合物10 (3.14 g, 0.01 mol)和DDQ (9.08 g, 0.04 mol))加入到盛有80 mL二氧六环的三口反应瓶中, 搅拌并加热至90 ℃, 反应结束后降温, 加入100 mL质量分数为8%的亚硫酸钠水溶液, 用乙酸乙酯(30 mL×2)萃取, 有机相干燥浓缩后得2.9 g (9.38 mmol) 4-氧代-2-苯基-4H-硫色酮-3-甲酸乙酯(11), 收率94%. 淡黄色固体, m.p. 95.4~97.5 ℃; 1H NMR (400 Hz, DMSO-d6) δ: 8.40 (dd, J=8, 1.2 Hz, 1H), 7.97 (d, J=8 Hz, 1H), 7.86~7.82 (m, 1H), 7.73~7.69 (m, 1H), 7.59~7.56 (m, 5H), 4.06 (q, J=7 Hz, 2H), 0.97 (t, J=7.2 Hz, 3H); 13C NMR (100 Hz, DMSO-d6) δ: 176.90, 165.38, 152.53, 136.91, 135.02, 133.30, 131.29, 130.60, 130.05, 129.56, 129.21, 128.49, 128.30, 127.38, 61.59, 14.07; HRMS (ESI) calcd for C18H15O3S [M+H]+ 311.0742, found 311.0743.
将上述中间体11 (3.1 g, 0.01 mol)投入到盛有30 mL无水乙醇的三口反应瓶中, 然后加入8 mL质量分数为10%的氢氧化钠水溶液, 加热至40 ℃水解2 h. 反应液减压浓缩除去大部分无水乙醇, 加入20 mL二氯甲烷, 用水(20 mL×2)萃取, 水相用质量分数为10%的盐酸酸化至pH<3, 再用二氯甲烷(20 mL×2)萃取. 有机相干燥、浓缩得2.5 g (0.009 mol) 4-氧代-2-苯基-4H-硫色酮- 3-甲酸(12), 白色固体, 收率90%. m.p. 220.4~221.4 ℃; 1H NMR (400 Hz, DMSO-d6) δ: 13.25 (s, 1H), 8.41 (dd, J=8.2, 1 Hz, 1H), 7.96 (d, J=8 Hz, 1H), 7.85~7.81 (m, 1H), 7.73~7.69 (m, 1H), 7.63~7.55 (m, 5H); 13C NMR (100 Hz, DMSO-d6) δ: 177.16, 166.86, 137.09, 135.37, 133.16, 132.01, 131.16, 130.11, 129.52, 129.14, 129.05, 128.51, 128.44, 127.31.
将化合物12 (564 mg, 2 mmol)和DCC (494.4 mg, 2.4 mmol))投入到盛有20 mL二氯甲烷的三口反应瓶中, 在0 ℃下滴加含有2.2 mmol各种取代胺的5 mL二氯甲烷溶液, 在此温度搅拌1 h后升温至30 ℃. 反应结束后过滤, 滤液倒入20 mL水中, 分液后取有机相浓缩, 残余物用薄层色谱分离(石油醚/乙酸乙酯, V∶V=2∶1), 得到目标化合物13a~13e.
4-氧代-N,N-二乙基-2-苯基-4H-硫色酮基-3-甲酰胺(13a): 淡红色粉末(纯度99%), 收率46%. m.p. 105.3~107.9 ℃; H NMR (400 Hz, DMSO-d6) δ: 8.40 (dd, J=8.2, 1 Hz, 1H), 7.95 (d, J=8 Hz, 1H), 7.84~7.80 (m, 1H), 7.71~7.67 (m, 1H), 7.60~7.49 (m, 5H), 3.55~3.47 (m, 1H), 3.21~3.12 (m, 1H), 2.99~2.87 (m, 2H), 0.83 (t, J=7.2 Hz, 3H), 0.72 (t, J=7 Hz, 3H); 13C NMR (100 Hz, DMSO-d6) δ: 177.39, 164.68, 149.36, 137.38, 135.09, 132.98, 130.91, 130.14, 129.17, 128.88, 128.68, 128.59, 127.23, 42.56, 38.11, 14.00, 12.25; HRMS (ESI) calcd for C20H20NO2S [M+H]+ 338.1215, found 338.1207.
N-烯丙基-4-氧代-2-苯基-4H-硫代色氨酸-3-甲酰胺(13b): 淡黄色粉末(纯度97%), 收率59%. m.p. 184.0~186.5 ℃; 1H NMR (400 Hz, DMSO-d6) δ: 8.42~8.34 (m, 2H), 7.93 ((d J=8 Hz, 1H), 7.83~7.79 (m, 1H), 7.71~7.67 (m, 1H), 7.60~7.48 (m, 5H), 5.56~5.48 (m, 1H), 4.91~4.85 (m, 2H), 3.63~3.60 (m, 2H); 13C NMR (100 Hz, DMSO-d6) δ: 177.58, 164.63, 150.32, 137.07, 135.64, 134.95, 134.26, 132.92, 130.73, 130.45, 129.14, 128.85, 128.66, 128.59, 127.19, 115.47, 41.28; HRMS (ESI) calcd for C19H16NO2S [M+H]+ 322.0902, found 322.0896.
N-(4-氯苄基)-4-氧代-2-苯基-4H-硫色酮基-3-甲酰胺(13c): 红棕色粉末(纯度97%), 收率38%. m.p. 255.1~258.0 ℃; 1H NMR (400 Hz, DMSO-d6) δ: 8.73 (t, J=6 Hz, 1H), 8.42 (dd, J=8, 1.2 Hz, 1H), 7.94 (dd, J=8, 0.8 Hz, 1H), 7.82~7.48 (m, 7H), 7.23~7.20 (m, 2H), 6.90 (d, J=8.4 Hz, 2H), 4.21 (d, J=6 Hz, 2H); 13C NMR (100 Hz, DMSO-d6) δ: 177.62, 164.96, 150.58, 138.36, 137.10, 135.46, 134.06, 132.99, 131.52, 130.79, 130.41, 129.25, 129.10, 128.92, 128.72, 128.61, 128.43, 127.22, 41.82; HRMS (ESI) calcd for C23H17ClNO2S [M+H]+ 406.0669, found 406.0663.
N-(4-氯苯基)-4-氧代-2-苯基-4H-硫色酮基-3-甲酰胺(13d): 红色粉末(纯度98%), 收率54%. m.p. 123.5~127.4 ℃; 1H NMR (400 Hz, DMSO-d6) δ: 10.48 (s, 1H), 8.44 (dd, J=8.2, 1 Hz, 1H), 7.99 (dd, J=8, 1 Hz, 1H), 7.85~7.61 (m, 4H), 7.50~7.46 (m, 5H), 7.33~7.31 (m, 2H); 13C NMR (100 Hz, DMSO-d6) δ: 177.62, 163.55, 151.44, 138.08, 137.04, 135.33, 133.87, 133.18, 130.98, 130.44, 129.35, 129.14, 129.10, 128.62, 128.44, 127.64, 127.32, 121.09; HRMS (ESI) calcd for C22H15ClNO2S [M+H]+ 392.0512, found 392.0504.
4-(4-氧代-2-苯基-4H-硫色酮基-3-甲酰胺基)苯甲酸异丙酯(13e): 红色粉末(纯度98%), 收率53%. m.p. 126.5~128.5 ℃; 1H NMR (400 Hz, DMSO-d6) δ: 10.71 (s, 1H), 8.45 (dd, J=8, 1.2 Hz, 1H), 7.99 (d, J=8 Hz, 1H), 7.88~7.84 (m, 3H), 7.75~7.71 (m, 1H), 7.64~7.58 (m, 4H), 7.50~7.48 (m, 3H), 5.13~5.07 (m, 1H), 1.29 (d, J=6.4 Hz, 6H); 13C NMR (100 Hz, DMSO-d6) δ: 177.65, 165.22, 163.99, 151.65, 143.33, 137.05, 135.26, 133.75, 133.21, 131.01, 130.67, 130.41, 129.37, 129.12, 128.61, 128.43, 127.33, 125.43, 118.91, 68.29, 22.16; HRMS (ESI) calcd for C26H22NO4S [M+H]+ 444.1270, found 444.1262.