Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (9): 3459-3481.DOI: 10.6023/cjoc202103032 Previous Articles Next Articles
Special Issue: 有机电合成虚拟专辑; 有机光催化虚拟合辑
REVIEWS
收稿日期:2021-03-19
修回日期:2021-04-04
发布日期:2021-06-07
通讯作者:
刘想
基金资助:
Xiang Liu( ), Wen Li, Canzhan Zhuang, Hua Cao
), Wen Li, Canzhan Zhuang, Hua Cao
Received:2021-03-19
Revised:2021-04-04
Published:2021-06-07
Contact:
Xiang Liu
Supported by:Share
Xiang Liu, Wen Li, Canzhan Zhuang, Hua Cao. Application of Photochemical/Electrochemical Synthesis in C—H Functionalization of Quinoxalin-2(1H)-one[J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3459-3481.
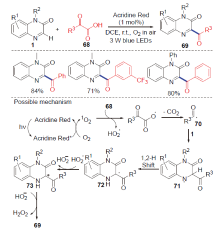
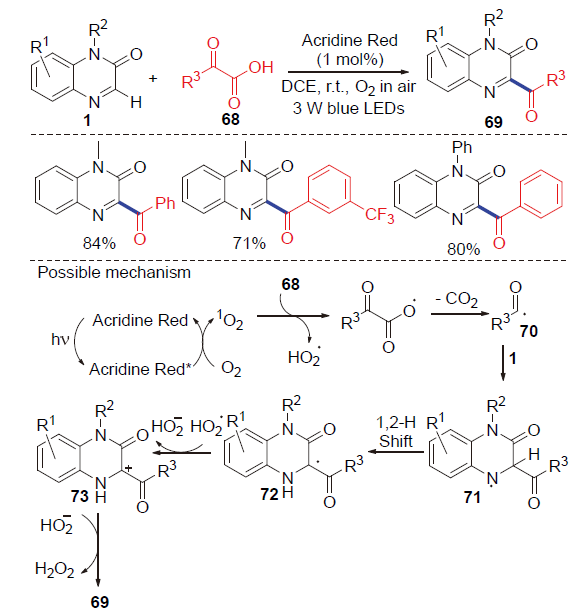
| [1] |
Yang, Y. C.; Zhang, S. Z.; Wu, B. B.; Ma, M. M.; Chen, X.; Qin, X. Y.; He, M. L.; Hussain, S.; Jing, C. J.; Ma, B.; Zhu, C. ChemMedChem 2012, 7, 823.
doi: 10.1002/cmdc.v7.5 pmid: 24793885 |
|
(b) Shi, L. L.; Hu, W.; Wu, J. F.; Zhou, H. Y.; Zhou, H.; Li, L. Mini-Rev. Med. Chem. 2018, 18, 392.
doi: 10.2174/1389557517666171101111134 pmid: 24793885 |
|
|
(c) Hussain, S.; Parveen, S.; Hao, X.; Zhang, S. Z.; Wang, W.; Qin, X. Y.; Yang, Y. C.; Chen, X.; Zhu, S. J.; Zhu, C. J.; Ma, B. Eur. J. Med. Chem. 2014, 80, 383.
doi: 10.1016/j.ejmech.2014.04.047 pmid: 24793885 |
|
|
(d) Shi, J. W.; Wei, W. Chin. J. Org. Chem. 2020, 40, 2170. (in Chinese).
doi: 10.6023/cjoc202000041 pmid: 24793885 |
|
|
( 时建伟, 魏伟, 有机化学, 2020, 40, 2170.)
pmid: 24793885 |
|
|
(e) Mao, P.; Zhu, J. L.; Yuan, J. W.; Liang, L. R.; Xiao, Y. M.; Zhang, C. S. Chin. J. Org. Chem. 2019, 39, 1529. (in Chinese).
doi: 10.6023/cjoc201904025 pmid: 24793885 |
|
|
( 毛璞, 朱军亮, 袁金伟, 杨亮茹, 肖咏梅, 张长森, 有机化学, 2019, 39, 1529.)
pmid: 24793885 |
|
|
(f) Hai, M.; Guo, L. N.; Wang, L.; Duan, X. H. Acta. Chim. Sinica 2019, 77, 895. (in Chinese).
doi: 10.6023/A19040155 pmid: 24793885 |
|
|
( 海曼, 郭丽娜, 王乐, 段新华, 化学学报, 2019, 77, 895.)
pmid: 24793885 |
|
| [2] |
(a) Weïwer, M.; Spoonamore, J.; Wei, J.; Guichard, B.; Ross, N. T.; Masson, K.; Silkworth, W.; Dandapani, S.; Palmer, M.; Scherer, C. A.; Stern, A. M.; Schreiber, S. L.; Munoz, B. ACS Med. Chem. Lett. 2012, 3, 1034.
doi: 10.1021/ml300246r pmid: 11170649 |
|
(b) Qin, X. Y.; Hao, X.; Han, H.; Zhu, S. J.; Yang, Y. C.; Wu, B. B.; Hussain, S.; Parveen, S.; Jing, C. J.; Ma, B.; Zhu, C. J. J. Med. Chem. 2015, 58, 1254.
doi: 10.1021/jm501484b pmid: 11170649 |
|
|
(c) Ajani, O. O. Eur. J. Med. Chem. 2014, 85, 688.
doi: 10.1016/j.ejmech.2014.08.034 pmid: 11170649 |
|
|
(d) Lawrence, D. S.; Copper, J. E.; Smith, C. D. J. Med. Chem. 2001, 44, 594.
pmid: 11170649 |
|
|
(e) Liu, Z.; Yu, S.; Chen, D.; Shen, G.; Wang, Y.; Hou, L.; Lin, D.; Zhang, J.; Ye, F. Drug Des., Dev. Ther. 2016, 10, 1489.
pmid: 11170649 |
|
| [3] |
(a) Qin, Y.; Zhu, L.; Luo, S. Chem. Rev. 2017, 117, 9433.
doi: 10.1021/acs.chemrev.6b00657 |
|
(b) Ping, L.; Chung, D. S.; Bouffard, J.; Lee, S. G. Chem. Soc. Rev. 2017, 46, 4299.
doi: 10.1039/C7CS00064B |
|
|
(c) Shen, J. B.; Xu, J.; Huang, L.; Zhu, Q.; Zhang, P. F. Adv. Synth. Catal. 2020, 362, 230.
doi: 10.1002/adsc.v362.1 |
|
|
(d) Yuan, J. W.; Liu, S. N.; Qiao, L. B. Adv. Synth. Catal. 2017, 359, 4197.
doi: 10.1002/adsc.v359.23 |
|
|
(e) Ramesh, B.; Reddy, C. R.; Kumar, G. R.; Reddy, B. V. S. Tetrahedron Lett. 2018, 59, 628.
doi: 10.1016/j.tetlet.2017.12.085 |
|
|
(f) Aganda, K. C. C.; Hong, B.; Lee, A. Adv. Synth. Catal. 2021, 363, 1443.
doi: 10.1002/adsc.v363.5 |
|
| [4] |
(a) Yuan, J. W.; Fu, J. H.; Yin, J. H.; Dong, Z. H.; Xiao, Y. M.; Mao, P.; Qu, L. B. Org. Chem. Front. 2018, 5, 2820.
doi: 10.1039/C8QO00731D |
|
(b) Wang, L. P.; Zhang, Y. C.; Li, F. F.; Hao, X. Y.; Zhang, H. Y.; Zhao, J. Q. Adv. Synth. Catal. 2018, 360, 3969.
doi: 10.1002/adsc.v360.20 |
|
|
(c) Hong, G. F.; Yuan, J. W.; Fu, J. H.; Pan, G. Y.; Wang, Z. W.; Yang, L. R.; Xiao, Y. M.; Mao, P.; Zhang, X. M. Org. Chem. Front. 2019, 6, 1173.
doi: 10.1039/C9QO00105K |
|
|
(d) Wang, J.; Li, J.; Wei, Y. Y.; Yang, J. Y.; Huo, C. D. Org. Chem. Front. 2018, 5, 3534.
doi: 10.1039/C8QO01049H |
|
|
(e) Ke, Q. M.; Yan, G. B.; Yu, J.; Wu, X. M. Org. Biomol. Chem. 2019, 17, 5863.
doi: 10.1039/C9OB00782B |
|
| [5] |
(a) He, K. H.; Tan, F. F.; Zhou, C. Z.; Zhou, G. J.; Yang, X. L.; Li, Y. Angew. Chem., nt. Ed. 2017, 56, 3080.
|
|
(b) Wang, H. M.; Gao, X. L.; Lv, Z. C.; Abdelilah, T.; Lei, A. W. Chem. Rev. 2019, 119, 6769.
doi: 10.1021/acs.chemrev.9b00045 |
|
|
(c) Yu, X. Y.; Chen, J. R.; Xiao, W. J. Chem. Rev. 2021, 121, 506.
doi: 10.1021/acs.chemrev.0c00030 |
|
|
(d) Mcatee, R. C.; Mcclain, E. J.; Stephenson, C. R. J. Trends Chem. 2019, 1, 111.
doi: 10.1016/j.trechm.2019.01.008 |
|
|
(d) Bhaskaran, R. P.; Babu, B. P. Adv. Synth. Catal. 2020, 362, 5219.
doi: 10.1002/adsc.v362.23 |
|
|
(e) Pan, X. L.; Xia, H. G.; Wu, J. Org. Chem. Front. 2016, 3, 1163.
|
|
|
(f) Shen, J. B.; Xu, J.; He, L.; Ouyang, Y.; Haung, L.; Li, W. M.; Zhu, Q.; Zhang, P. F. Org. Lett. 2021, 23, 1204.
doi: 10.1021/acs.orglett.0c04148 |
|
|
(g) Xu, J.; Huang, L.; He, L.; Ni, Z. G.; Shen, J. B.; Li, X. L.; Chen, K. X.; Li, W. M.; Zhang, P. F. Green Chem. 2021, 23, 2123.
doi: 10.1039/D0GC04235H |
|
| [6] |
(a) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 7, 5322.
|
|
(b) Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 17, 10075.
|
|
|
(c) Hari, D. P.; König, B. Chem. Commun. 2014, 50, 6688.
doi: 10.1039/C4CC00751D |
|
| [7] |
(a) Jiang, Y. Y.; Xu, K.; Zeng, C. C. Chem. Rev. 2018, 9, 4485.
|
|
(b) Waldvogel, S. R.; Lips, S.; Selt, M.; Riehl, B.; Kampf, C. J. Chem. Rev. 2018, 118, 6706.
doi: 10.1021/acs.chemrev.8b00233 |
|
|
(c) Pegis, M. L.; Wise, C. F.; Martin, D. J.; Mayer, J. M. Chem. Rev. 2018, 5, 2340.
|
|
|
(d) Wang, H. Q.; Xu, W. T.; Xin, L. L.; Liu, W. M.; Wang, Z. Q.; Xu, K. J. Org. Chem. 2016, 81, 3681.
doi: 10.1021/acs.joc.6b00343 |
|
| [8] |
(a) Cui, Z. M.; Zhu, B. F.; Li, X. C.; Cao, H. Org. Chem. Front. 2018, 5, 2219.
doi: 10.1039/C8QO00443A |
|
(b) Yu, Y.; Su, Z. Q.; Cao, H. Chem. Rec. 2019, 19, 2105.
doi: 10.1002/tcr.v19.10 |
|
|
(c) Cao, H.; Lei, S.; Li, N. Y.; Chen, L. B.; Liu, J. Y.; Cai, H. Y.; Qiu, S. X.; Tan, J. W. Chem. Commun. 2015, 51, 1823
doi: 10.1039/C4CC09134E |
|
|
(d) Lei, S.; Cao, H.; Chen, L. B.; Liu, J. Y.; Cai, H. Y. Adv. Synth. Catal. 2015, 357, 3109.
doi: 10.1002/adsc.201500391 |
|
| [9] |
(a) Brahmachari, G. RSC Adv. 2016, 6, 64676.
doi: 10.1039/C6RA14399G |
|
(b) Resch, V.; Schrittwieser, J. H.; Siirola, E.; Kroutil, W. Curr. Opin. Biotechnol. 2011, 22, 793.
doi: 10.1016/j.copbio.2011.02.002 |
|
|
(c) Yamaguchi, J.; Yamaguchi, A. D.; Itami, K. Angew. Chem., Int. Ed. 2012, 51, 8960.
doi: 10.1002/anie.201201666 |
|
| [10] |
Wei, W.; Wang, L. L.; Yue, H. L.; Bao, P. L.; Liu, W. W.; Hu, C. S.; Yang, D. S.; Wang, H. ACS Sustainable Chem. Eng. 2018, 6, 17252.
doi: 10.1021/acssuschemeng.8b04652 |
| [11] |
Zhang, W.; Pan, Y. L.; Yang, C.; Chen, L.; Li, X.; Cheng, J. P. J. Org. Chem. 2019, 84, 7786.
doi: 10.1021/acs.joc.9b00657 pmid: 31140803 |
| [12] |
Zhang, W.; Pan, Y. L.; Yang, C.; Li, X.; Wang, B. Org. Chem. Front. 2019, 6, 2765.
doi: 10.1039/c9qo00625g |
| [13] |
Zhao, B.; Kong, X. Q.; Xu, B. Tetrahedron Lett. 2019, 60, 2063.
doi: 10.1016/j.tetlet.2019.06.059 |
| [14] |
Zhang, H. D.; Xu, J.; Zhao, J. M.; Zhang, P. F.; Li, W. M. Org. Biomol. Chem. 2019, 17, 10201.
doi: 10.1039/C9OB02203A |
| [15] |
Liu, L. X.; Pan, N.; Sheng, W.; Su, L. B.; Liu, L.; Dong, J. Y.; Zhou, Y. B.; Yin, S. F. Adv. Synth. Catal. 2019, 361, 4126.
doi: 10.1002/adsc.v361.17 |
| [16] |
Yan, Z. Y.; Sun, B.; Zhang, X.; Zhuang, X. H.; Yang, J.; Su, W. K.; Jin, C. Chem.-Asian J. 2019, 14, 3344.
doi: 10.1002/asia.v14.19 |
| [17] |
Xue, W. X.; Su, Y. P.; Wang, K. H.; Zhang, R.; Feng, Y. W.; Cao, L. D.; Huang, D. F.; Hu, Y. L. Org. Biomol. Chem. 2019, 17, 6654.
doi: 10.1039/C9OB01169B |
| [18] |
Xie, L. Y.; Jiang, L. L.; Tan, J. X.; Wang, Y.; Xu, X. Q.; Zhang, B.; Cao, Z.; He, W. M. ACS Sustainable Chem. Eng. 2019, 7, 14153.
doi: 10.1021/acssuschemeng.9b02822 |
| [19] |
Mane, K. D.; Kamble, R. B.; Suryavanshi, G. New J. Chem. 2019, 43, 7403.
doi: 10.1039/C9NJ00075E |
| [20] |
Wei, Z. J.; Qi, S. J.; Xu, Y. H.; Liu, H.; Wu, J. Z.; Li, H. S.; Xia, C. C.; Duan, G. Y. Adv. Synth. Catal. 2019, 361, 5490.
doi: 10.1002/adsc.v361.23 |
| [21] |
Zheng, D. Q.; Studer, A. Org. Lett. 2019, 21, 325.
doi: 10.1021/acs.orglett.8b03849 |
| [22] |
Wang, J. Y.; Sun, B.; Zhang, L.; Xu, T. W.; Xie, Y. Y.; Jin, C. Asian J. Org. Chem. 2019, 8, 1942.
doi: 10.1002/ajoc.v8.10 |
| [23] |
Jin, C.; Zhuang, X. C.; Sun, B.; Li, D. Y.; Zhu, R. Asian J. Org. Chem. 2019, 8, 1490.
doi: 10.1002/ajoc.v8.8 |
| [24] |
He, X. K.; Lu, J.; Zhang, A. J.; Zhang, Q. Q.; Xu, G. Y.; Xuan, J. Org. Lett. 2020, 22, 5984.
doi: 10.1021/acs.orglett.0c02080 |
| [25] |
Zhang, W.; Xiang, X. X.; Chen, J. Y.; Yang, C.; Pan, Y. L.; Cheng, J. P.; Meng, J. B.; Li, X. Nat. Commun. 2020, 11, 638.
doi: 10.1038/s41467-020-14494-8 pmid: 32005825 |
| [26] |
Gao, Y. H.; Zhao, L. L.; Xiang, T. Y.; Li, P. H.; Wang, L. RSC Adv. 2020, 10, 10559.
doi: 10.1039/D0RA02059A |
| [27] |
Wang, L. L.; Bao, P. L.; Liu, W. W.; Liu, S. T.; Hu, C. S.; Yue, H. L.; Yang, D. S.; Wei, W. Chin. J. Org. Chem. 2018, 38, 3189. (in Chinese).
doi: 10.6023/cjoc201807014 |
|
( 王雷雷, 鲍鹏丽, 刘维伟, 刘思彤, 胡昌松, 岳会兰, 杨道山, 魏伟, 有机化学, 2018, 38, 3189.)
|
|
| [28] |
Kwon, S. J.; Jung, H. I.; Kim, D. Y. ChemistrySelect 2018, 3, 5824.
doi: 10.1002/slct.v3.21 |
| [29] |
Bao, P. L.; Liu, F.; Lv, Y. F.; Yue, H. L.; Li, J. S.; Wei, W. Org. Chem. Front. 2019, 2020, 492.
|
| [30] |
Xie, L. Y.; Bai, Y. S.; Xu, X. Q.; Xia, P.; Tang, H. S.; Huang, Y.; Lin, Y. W.; Cao, Z.; He, W. M. Green Chem. 2020, 22, 1720.
doi: 10.1039/C9GC03899J |
| [31] |
Lu, J.; He, X. K.; Cheng, X.; Zhang, A.; Xu, G. Y.; Xuan, J. Adv. Synth. Catal. 2020, 362, 2179.
|
| [32] |
Xie, L.Y.; Peng, S.; Peng, L. H.; Peng, C.; Lin, Y. W.; Yu, X. Y.; Cao, Z.; Peng, Y. Y.; He, W. M. Green Chem. 2021, 23, 374.
doi: 10.1039/D0GC02844D |
| [33] |
Wei, W.; Wang, L. L.; Bao, P. L.; Shao, Y.; Yue, H. L.; Yang, D. S.; Yang, X. B.; Zhao, X. H.; Wang, H. Org. Lett. 2018, 20, 7125.
doi: 10.1021/acs.orglett.8b03079 pmid: 30372088 |
| [34] |
Xie, L. Y.; Hu, J. L.; Song, Y. X.; Jia, G. K.; Lin, Y. W.; He, J. Y.; Cao, Z.; He, W. M. ACS Sustainable Chem. Eng. 2019, 7, 19993.
doi: 10.1021/acssuschemeng.9b05715 |
| [35] |
Sun, M. L.; Wang, L.; Zhao, L. L.; Wang, Z. M.; Li, P. H. ChemCatChem 2020, 12, 5261.
doi: 10.1002/cctc.v12.20 |
| [36] |
Zhao, L. L.; Wang, L.; Gao, Y. H.; Wang, Z. M.; Li, P. H. Adv. Synth. Catal. 2019, 361, 1.
doi: 10.1002/adsc.v361.1 |
| [37] |
Zhou, J. D.; Zhou, P.; Zhao, T. T.; Ren, Q. L.; Li, J. J. Adv. Synth. Catal. 2019, 361, 5371.
doi: 10.1002/adsc.v361.23 |
| [38] |
Xu, X. B.; Xia, C. C.; Li, X. J.; Sun, J.; Hao, L. Q. RSC Adv. 2020, 10, 2016.
doi: 10.1039/C9RA10194B |
| [39] |
Xie, L. Y.; Liu, Y. S.; Ding, H. R.; Gong, S.; Tan, J. X.; He, J. Y.; Gao, Z.; He, W. M. Chin. J. Catal. 2020, 41, 1168.
doi: 10.1016/S1872-2067(19)63526-6 |
| [40] |
Teng, Q. H.; Yao, Y.; Wei, W. X.; Tang, H. T.; Li, J. R.; Pan, Y. M. Green Chem. 2019, 21, 6241.
doi: 10.1039/C9GC03045J |
| [41] |
Xie, L. Y.; Chen, Y. L.; Qin, L.; Wen, Y.; Xie, J. W.; Tan, J. X.; Huang, Y.; Cao, Z.; He, W. M. Org. Chem. Front. 2019, 6, 3950.
doi: 10.1039/C9QO01240K |
| [42] |
Kim, Y.; Kim, D. Y. Tetrahedron Lett. 2018, 59, 2443.
doi: 10.1016/j.tetlet.2018.05.034 |
| [43] |
Dai, C. H.; Zhan, Y. L.; Liu, P.; Sun, P. P. Green Chem. 2021, 23, 314.
doi: 10.1039/D0GC03697H |
| [44] |
Dou, G. Y.; Jiang, Y. Y.; Xu, K.; Zeng, C. C. Org. Chem. Front. 2019, 6, 2392.
doi: 10.1039/C9QO00552H |
| [45] |
Niu, K. K.; Song, L. Y.; Hao, Y. K.; Wang, Q. M. Chem. Commun. 2020, 56, 11673.
doi: 10.1039/D0CC05391K |
| [46] |
Gao, Y. Y.; Wu, Z. G.; Yu, L.; Wang, Y.; Pan, Y. Angew. Chem., Int. Ed. 2020, 59, 10859.
doi: 10.1002/anie.v59.27 |
| [47] |
Wen, J. W.; Yang, X. T.; Yan, K. L.; Qin, H. Y.; Ma, J.; Sun, X. J.; Yang, J. J.; Wang, H. Org. Lett. 2021, 23, 1081.
doi: 10.1021/acs.orglett.0c04296 |
| [48] |
Niu, K. K.; Hao, Y. K.; Song, L. Y.; Liu, Y. X.; Wang, Q. M. Green Chem. 2021, 23, 302.
doi: 10.1039/D0GC03892J |
| [49] |
Li, K. J.; Xu, K.; Liu, Y. G.; Zeng, C. C.; Sun, B. G. Adv. Synth. Catal. 2018, 361, 1033.
doi: 10.1002/adsc.v361.5 |
| [50] |
Jiang, X. P.; Yang, L. C.; Ye, Z. H.; Du, X. F.; Fang, L. Y.; Zhu, Y.; Chen, K. D.; Li, J. J.; Yu, C. M. Eur. J. Org. Chem. 2020, 2020, 1687.
|
| [51] |
Zhou, J. D.; Li, Z. H.; Sun, Z. X.; Ren, Q. L.; Zhang, Q. W.; Li, H.; Li, J. J. J. Org. Chem. 2020, 85, 4365.
doi: 10.1021/acs.joc.0c00050 |
| [52] |
Li, K. J.; Jiang, Y. Y.; Xu, K.; Zeng, C. C.; Sun, B. G. Green Chem. 2019, 21, 4412.
doi: 10.1039/C9GC01474H |
| [1] | Hong'en Tong, Hongyu Guo, Rong Zhou. Progress on Visible-Light Promoted Addition Reactions of Inert C—H Bonds to Carbonyls [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 54-69. |
| [2] | Min Wu, Bo Liu, Jialong Yuan, Qiang Fu, Rui Wang, Dawei Lou, Fushun Liang. Recent Progress in the C—S Bond Formation Reactions Mediated by Visible Light [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2269-2292. |
| [3] | Zhou Zhang, Yu Guo, Jing Yang, Dan Wu, Jiaxin Wang, Xinyue Hong, Peijun Cai, Liangce Rong. Electrochemically Promoted Halogenation of Imidazoland-[1,2-a]pyridine with Dichloro(bromo)ethylene and Iodoform [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2104-2109. |
| [4] | Linlin Du, Hua Zhang. Photochemical and Electrochemical Borylation Involving Aryl and Alkyl Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1726-1741. |
| [5] | Juan Tang, Jiayu Hu, Zhiqiang Zhu, Shouzhi Pu. Recent Advances in Visible-Light-Induced Organic Phosphine- Promoted Deoxygenative Functionalization Reactions [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4036-4056. |
| [6] | Changyuan Du, Yucai Tang, Jinglin Duan, Biyu Yang, Yupeng He, Qian Zhou, Xuewen Liu. Organic-Dye-Catalyzed Visible-Light-Mediated Alkoxycarbon-ylation of 2-Aryl-N-acryloyl Indoles with Carbazates [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4268-4276. |
| [7] | Wanjie Wei, Lei Zhan, Lei Gao, Guobao Huang, Xianli Ma. Research Progress of Electrochemical Synthesis of C-Sulfonyl Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 17-35. |
| [8] | Panpan Lei, Qinlin Chen, Hang Chen, Yang Zhou, Linhai Jin, Wei Wang, Fener Chen. Synthesis of Bibenzyl Derivatives via Visible-Light-Promoted 1,5-Hydrogen Atom Transfer/Radical Coupling Reactions of N-Fluorocarboxamides [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 254-264. |
| [9] | Haoyang Liu, Shuangshuang Sun, Xianli Ma, Yanyan Chen, Yanli Xu. Synthesis of Selenylated Spiro[indole-3,3'-quinoline] Derivatives via Visible-Light-Promoted Isocyanide Insertion [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2867-2876. |
| [10] | Zhentao Pan, Tong Liu, Yongmin Ma, Jianbo Yan, Ya-Jun Wang. Construction of Quinazolin(thi)ones by Brønsted Acid/Visible-Light Photoredox Relay Catalysis [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2823-2831. |
| [11] | Ruisheng Liu, Shuangmin Fu, Xiumin Chu, Lingli Zhang, Rou Ding, Xian'en Zhao, Huilan Yue, Wei Wei. Visible-Light-Induced Denitrification Oxygenation Reaction of α-Diazoesters to Construct α-Oxyimido Esters [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2462-2470. |
| [12] | Yazhou Wang, Yuhang Zhu, Lixia Xu, Rui He, Jian Zhang. Recent Advances in Geminal-Group-Directed Alkenyl C—H Functionalization [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2000-2014. |
| [13] | Runye Gao, Lingling Zuo, Fang Wang, Chuanying Li, Huajiang Jiang, Pinhua Li, Lei Wang. Recent Advances in Controllable Organic Reactions Induced by Visible Light without External Photocatalyst [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 1883-1903. |
| [14] | Yubing Shi, Wenji Bai, Weihua Mu, Jiangping Li, Jiawei Yu, Bing Lian. Research Progress on Density Functional Theory Study of Palladium-Catalyzed C—H Functionalization to Form C—X (X=O, N, F, I, …) Bonds [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1346-1374. |
| [15] | Xin Sun, Chaofan Qu, Chaorui Ma, Xiaowei Zhao, Guobi Chai, Zhiyong Jiang. Photoredox Catalytic Cascade Radical Addition to Construct 1,4- Diketone-Functionalized Quinoxalin-2(1H)-one Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1396-1406. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||