Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (7): 2236-2242.DOI: 10.6023/cjoc202202038 Previous Articles Next Articles
ARTICLES
王泽坤a, 徐子悦a, 李娟娟b, 余尚博c, 王辉a, 郭东升b, 张丹维a, 黎占亭a,c,*( )
)
收稿日期:2022-02-28
修回日期:2022-03-13
发布日期:2022-08-09
通讯作者:
黎占亭
基金资助:
Ze-Kun Wanga, Zi-Yue Xua, Juan-Juan Lib, Shang-Bo Yuc, Hui Wanga, Dong-Sheng Guob, Dan-Wei Zhanga, Zhan-Ting Lia,c( )
)
Received:2022-02-28
Revised:2022-03-13
Published:2022-08-09
Contact:
Zhan-Ting Li
Supported by:Share
Ze-Kun Wang, Zi-Yue Xu, Juan-Juan Li, Shang-Bo Yu, Hui Wang, Dong-Sheng Guo, Dan-Wei Zhang, Zhan-Ting Li. Gradient Enhancement of Supramolecular Organic Framework for Solubilization of Hydrophobic Molecules by Two Molecular Containers in Water[J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2236-2242.
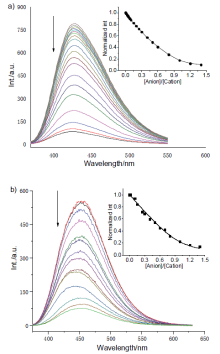
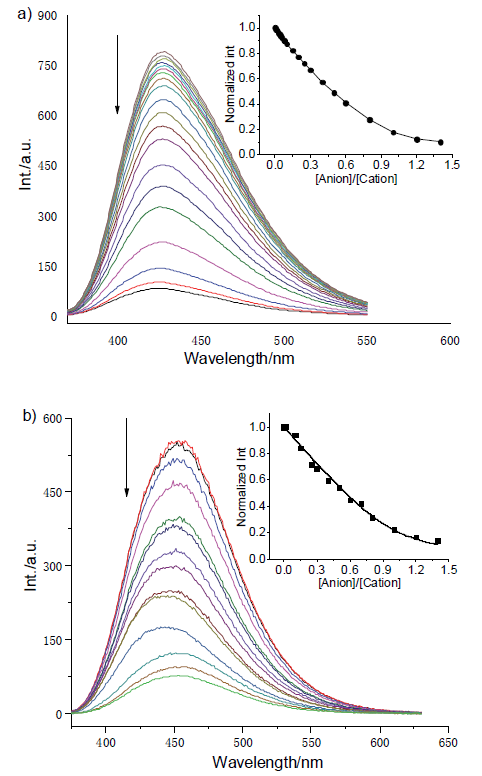
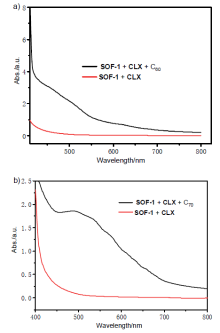
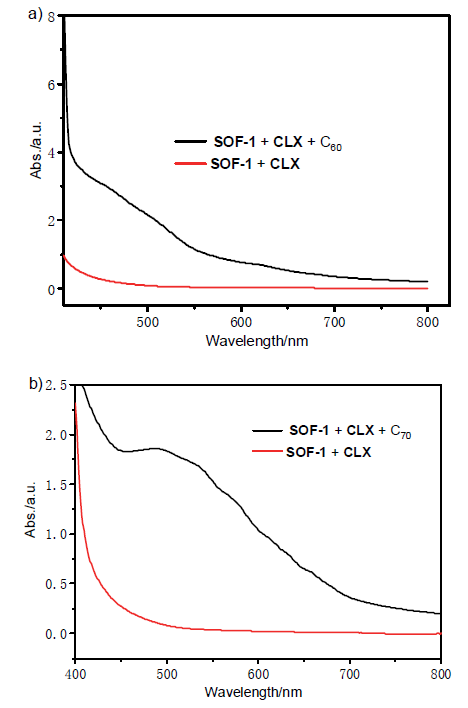


| [1] |
(a) Brewster, M. E.; Loftsson, T. Adv. Drug Delivery Rev. 2007, 59, 645.
doi: 10.1016/j.addr.2007.05.012 |
|
(b) Rao, V. M.; Stella, V. J. J. Pharm. Sci. 2003, 92, 927.
doi: 10.1002/jps.10341 |
|
| [2] |
(a) Hu, Y.; Qiu, C.; Qin, Y.; Xu, X.; Fan, L. Wang, J.; Jin, Z. Trend. Food Sci. Technol. 2021, 109, 398.
doi: 10.1016/j.tifs.2020.12.023 |
|
(b) Jambhekar, S. S.; Breen, P. Drug Delivery Today 2016, 21, 363.
|
|
|
(c) Qie, S.; Hao, Y.; Liu, Z.; Wang, J.; Xi, J. Acta Chim. Sin. 2020, 78, 232. (in Chinese)
|
|
|
( 郄淑燕, 郝莹, 刘宗建, 王锦, 席家宁, 化学学报, 2020, 78, 232.)
doi: 10.6023/A20010006 |
|
| [3] |
(a) Nakahata, M.; Takashima, Y.; Harada, A. Chem. Pharm. Bull. 2017, 65, 330.
doi: 10.1248/cpb.c16-00778 |
|
(b) Qi, W.; Ma, C.; Yan, Y.; Huang, J. Curr. Opin. Colloid Interface Sci. 2021, 56, 101526.
doi: 10.1016/j.cocis.2021.101526 |
|
|
(c) Xu, W.; Li, X.; Wang, L.; Li, S.; Chu, S.; Wang, J.; Li, Y.; Hou, J.; Luo, Q.; Liu, J. Front. Chem. 2021, 9, 635507.
doi: 10.3389/fchem.2021.635507 |
|
|
(d) Zhou, W.-L.; Chen, Y.; Liu, Y. Acta Chim. Sin. 2020, 78, 1164. (in Chinese)
|
|
|
( 周维磊, 陈湧, 刘育, 化学学报, 2020, 78, 1164.)
doi: 10.6023/A20100486 |
|
| [4] |
(a) Yu, Y.; Rebek, R. Acc. Chem. Res. 2018, 51, 3031.
doi: 10.1021/acs.accounts.8b00269 |
|
(b) Zhu, H.; Li, Q.; Khalil-Cruz, L. E.; Khashab, N. M.; Yu, G.; Huang, F. Sci. China Chem. 2021, 64, 688.
doi: 10.1007/s11426-020-9932-9 |
|
|
(c) Yin, H.; Wang, R. Isr. J. Chem. 2018, 58, 188.
doi: 10.1002/ijch.201700092 |
|
|
(d) Gu, A.; Wheate, N. J. J. Inclus. Phenom. Macrocycl. Chem. 2021, 100, 55.
doi: 10.1007/s10847-021-01055-9 |
|
|
(e) Yang, K.; Zhang, Z.; Du, J.; Li, W.; Pei, Z. Chem. Commun. 2020, 56, 5865.
doi: 10.1039/D0CC02001J |
|
|
(f) Zheng, Z.; Geng, W.-C.; Xu, Z.; Guo, D.-S. Isr. J. Chem. 2019, 59, 913.
doi: 10.1002/ijch.201900032 |
|
|
(g) Yang, X.; Chen, M.; Wang, F.; Jin, X.-Y.; Cong, H.; Tao, Z. Mini-Rev. Org. Chem. 2018, 15, 274.
doi: 10.2174/1570193X15666171228150315 |
|
|
(h) Sathiyajith, C.; Shaikh, R. R.; Han, Q.; Zhang, Y.; Meguellati, K.; Yang, Y.-W. Chem. Commun. 2017, 53, 677.
doi: 10.1039/C6CC08967D |
|
|
(i) Yin, H.; Bardelang, D.; Wang, R. Trends Chem. 2021, 3, 1.
doi: 10.1016/j.trechm.2020.08.008 |
|
|
(j) Li, Z.; Wang, H.; Zhang, D. Chem. J. Chin. Univ. 2020, 41, 1139. (in Chinese)
|
|
|
( 黎占亭, 王辉, 张丹维, 高等学校化学学报, 2020, 41, 1139.)
|
|
|
(k) Shi, Q.; Wang, X.; Liu, B.; Qiao, P.; Li, J.; Wang, L. Chem. Commun. 2021, 57, 12379.
doi: 10.1039/D1CC04400A |
|
|
(l) Zhang, S.; Boussouar, I.; Li, H. Chin. Chem. Lett. 2021, 32, 642.
doi: 10.1016/j.cclet.2020.06.035 |
|
|
(m) Li, S.; Gao, Y.; Ding, Y.; Xu, A.; Tan, H. Chin. Chem. Lett. 2021, 32, 313.
doi: 10.1016/j.cclet.2020.04.049 |
|
|
(n) Xiao, T.; Zhou, L.; Sun, X.-Q.; Huang, F.; Lin, C.; Wang, L. Chin. Chem. Lett. 2020, 31, 1.
doi: 10.1016/j.cclet.2019.05.011 |
|
|
(o) Li, R.-H.; Ma, J.; Sun, Y.; Li, H. Chin. Chem. Lett. 2020, 31, 3095.
doi: 10.1016/j.cclet.2020.06.041 |
|
|
(p) Duan, Z.; Xu, F.; Huang, X.; Qian, Y.; Li, H.; Tian, W. Macromol. Rapid Commun. 2021, e2100775.
|
|
| [5] |
(a) Hussain, A. M.; Ashraf, U. M.; Muhammad, G.; Tahir, N. M.; Bukhari, N. A. S. Curr. Pharm. Des. 2017, 23, 2377.
|
|
(b) Pan, Y. C.; X. Y. Hu, X. Y.; Guo, D.-S. Angew. Chem., Int. Ed. 2021, 60, 2768.
doi: 10.1002/anie.201916380 |
|
|
(c) Li, P.; Chen, Y.; Liu, Y. Chin. Chem. Lett. 2019, 30, 1190.
doi: 10.1016/j.cclet.2019.03.035 |
|
|
(d) Guo, D.-S.; Liu, Y. Acc. Chem. Res. 2014, 47, 1925.
doi: 10.1021/ar500009g |
|
|
(e) Perret, F.; Coleman, A. W. Chem. Commun. 2011, 47, 7303.
doi: 10.1039/c1cc11541c |
|
|
(f) Zhou, Y.; Li, H.; Yang, Y.-W. Chin. Chem. Lett. 2015, 26, 825.
doi: 10.1016/j.cclet.2015.01.038 |
|
| [6] |
(a) Macartney, D. H. Future Med. Chem. 2013, 5, 2075.
doi: 10.4155/fmc.13.164 pmid: 24215347 |
|
(b) Yin, H.; Zhang, X.; Wei, J.; Lu, S.; Bardelang, D.; Wang, R. Theranostics 2021, 11, 1513.
doi: 10.7150/thno.53459 pmid: 24215347 |
|
|
(c) Ma, D.; Hettiarachchi, G.; Nguyen, D.; Zhang, B.; Wittenberg, J. B.; Zavalij, P. Y.; Briken, V.; Isaacs, L. Nat. Chem. 2012, 4, 503.
doi: 10.1038/nchem.1326 pmid: 24215347 |
|
|
(d) Mao, D.; Liang, Y.; Liu, Y.; Zhou, X.; Ma, J.; Jiang, B.; Liu, J.; Ma, D. Angew. Chem. Int. Ed. 2017, 56, 12614.
doi: 10.1002/anie.201707164 pmid: 24215347 |
|
|
(e) Liu, J.; Chen, L.; Dong, G.; Yang, J.; Zhu, P.; Liao, X.; Wang, B.; Yang, B. J. Inclus. Phenom. Macrocycl. Chem. 2021, 100, 197.
doi: 10.1007/s10847-021-01073-7 pmid: 24215347 |
|
|
(f) Deng, C.-L.; Murkli, S. L.; Isaacs, L. Chem. Soc. Rev. 2020, 49, 7516-7532.
doi: 10.1039/D0CS00454E pmid: 24215347 |
|
|
(g) Lu, X.; Isaacs, L. Angew. Chem. Int. Ed. 2016, 55, 8076-8080.
doi: 10.1002/anie.201602671 pmid: 24215347 |
|
| [7] |
(a) Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. Angew. Chem., Int. Ed. 2005, 44, 4844.
doi: 10.1002/anie.200460675 |
|
(b) Barrow, S. J.; Kasera, S.; Rowland, M. J.; del Barrio, J.; Scherman, O. A. Chem. Rev. 2015, 115, 12320.
doi: 10.1021/acs.chemrev.5b00341 |
|
|
(c) Kim, K. Selvapalam, N.; Ko, Y. H.; Park, K. M.; Kim, D.; Kim, J. Chem. Soc. Rev. 2007, 36, 267.
doi: 10.1039/B603088M |
|
|
(d) Yao, Y. Q.; Chen, K.; Hua, Z. Y.; Zhu, Q. J.; Xue, S. F.; Tao, Z. J. Inclusion Phenom. Macrocyclic Chem. 2017, 89, 1.
doi: 10.1007/s10847-017-0733-5 |
|
| [8] |
(a) Yu, S.-B.; Lin, F.; Tian, T.; Yu, J.; Zhang, D.-W.; Li, Z.-T. Chem. Soc. Rev. 2022, 51, 434.
doi: 10.1039/D1CS00862E pmid: 25470406 |
|
(b) Tian, J.; Chen, L.; Zhang, D.-W.; Liu, Y.; Li, Z.-T. Chem. Commun. 2016, 52, 6351.
doi: 10.1039/C6CC02331B pmid: 25470406 |
|
|
(c) Tian, J.; Wang, H.; Zhang, D.-W.; Liu, Y.; Li, Z.-T. Natl. Sci. Rev. 2017, 4, 426.
doi: 10.1093/nsr/nwx030 pmid: 25470406 |
|
|
(d) Yang, B.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Chin. J. Chem. 2020, 38, 970.
doi: 10.1002/cjoc.202000085 pmid: 25470406 |
|
|
(e) Tian, J.; Zhou, T.-C.; Zhang, S.-C.; Aloni, S.; Altoe, M. V.; Xie, S.-H.; Wang, H.; Zhang, D.-W.; Zhao, X.; Liu, Y.; Li, Z.-T. Nat. Commun. 2014, 5, 5574.
doi: 10.1038/ncomms6574 pmid: 25470406 |
|
|
(f) Wang, H.; Zhang, D.; Li, Z. Chem. J. Chin. Univ. 2020, 41, 1139. (in Chinese)
pmid: 25470406 |
|
|
( 王辉, 张丹维, 黎占亭, 高等学校化学学报, 2020, 41, 1139.)
pmid: 25470406 |
|
| [9] |
(a) Xu, Z.-Y.; Mao, W.; Zhao, Z.; Wang, Z.-K.; Liu, Y.-Y.; Wu, Y.; Wang, H.; Zhang, D.-W.; Li, Z.-T.; Ma, D. J. Mater. Chem. B 2022, 10, 899.
doi: 10.1039/D1TB02601A |
|
(b) Liu, Y.; Liu, C.-Z.; Wang, Z.-K.; Zhou, W.; Wang, H.; Zhang, Y.-C.; Zhang, D.-W.; Ma, D.; Li, Z.-T. Biomaterials 2022, 284, 121467.
doi: 10.1016/j.biomaterials.2022.121467 |
|
| [10] |
(a) Yao, C.; Tian, J.; Wang, H.; Zhang, D.-W.; Liu, Y.; Zhang, F.; Li, Z.-T. Chin. Chem. Lett. 2017, 28, 893.
doi: 10.1016/j.cclet.2017.01.005 |
|
(b) Tian, J.; Yao, C.; Yang, W.-L.; Zhang, L.; Zhang, D.-W.; Wang, H.; Zhang, F.; Liu, Y.; Li, Z.-T. Chin. Chem. Lett. 2017, 28, 798.
doi: 10.1016/j.cclet.2017.01.010 |
|
|
(c) Zhang, Y.-C.; Zeng, P.-Y.; Ma, Z.-Q.; Xu, Z.-Y.; Wang, Z.-K.; Guo, B.; Yang, F.; Li, Z.-T. Drug Delivery 2022, 29, 128.
doi: 10.1080/10717544.2021.2021325 |
|
| [11] |
Yang, B.; Zhang, X.-D.; Li, J.; Tian, J.; Wu, Y.-P.; Yu, F.-X.; Wang, R.; Wang, H.; Zhang, D.-W.; Liu, Y.; Zhou, L.; Li, Z.-T. CCS Chem. 2019, 1, 156.
doi: 10.31635/ccschem.019.20180011 |
| [12] |
(a) Guo, D.-S.; Jiang, B.-P.; Wang, X.; Liu, Y. Org. Biomol. Chem. 2012, 10, 720.
doi: 10.1039/C2OB06973C |
|
(b) Ma, D.; Zavalij, P. Y.; Isaacs, L. J. Org. Chem. 2010, 75, 4786.
doi: 10.1021/jo100760g |
|
| [13] |
Yu, S.-B.; Qi, Q.; Yang, B.; Wang, H.; Zhang, D.-W.; Liu, Y.; Li, Z.-T. Small 2018, 14, 1801037.
|
| [14] |
Haino, T.; Yanase, M.; Fukunaga, C.; Fukazawa, Y. Tetrahedron 2006, 62, 2025.
doi: 10.1016/j.tet.2005.07.121 |
| [15] |
(a) Sun, W.; Wang, Y.; Ma, L.; Zheng, L.; Fang, W.; Chen, X.; Jiang, H. J. Org. Chem. 2018, 83, 14667.
doi: 10.1021/acs.joc.8b02674 |
|
(b) Han, Y.; Tian, Y.; Li, Z.; Wang, F. Chem. Soc. Rev. 2018, 47, 5165.
doi: 10.1039/C7CS00802C |
|
| [16] |
Hardie, M. J. Supramol. Chem. 2002, 14, 7.
doi: 10.1080/10610270290006529 |
| [17] |
Lim, S.; Pang, Z.; Hweiyuin, T.; Shaikh, M.; Adinarayana, G.; Garg, S. Drug Dev. Ind. Pharm. Drug Develop. Ind. Pharm. 2015, 41, 1.
|
| [1] | Lijun Xu, Zongjun Li, Fushe Han, Xiang Gao. N,N-Dimethylformamide-Promoted Synthesis of Fullerene-Fused Oxazoline Derivatives [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 242-250. |
| [2] | Xin Zuo, Shinuo Xu, Zhongyang Chen, Jianfeng Yan, Yaofeng Yuan. Research Progress of Electron Transport Properties in Ferrocene- Containing Single-Molecule Junctions [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2313-2322. |
| [3] | Lu Cheng, Fei Zeng, Xiaofeng Wang. Study on the Complexation Properties of Promellitic Diimide- Extended Pillar[6]aren and Carboxylate Guests [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 352-356. |
| [4] | Li Dai, Di Xu, Yifei Mao, Jiaqi Zhu, Mengjiao Yang. Structures and Synthetic Strategies of Chiral Oxazolinyl Ferrocene Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2364-2375. |
| [5] | Jingjing Guo, Minjie Guo. Progress in Design and Application of Supramolecular Fluorescent Systems Based on Difluoroboron-Dipyrromethene and Macrocyclic Compounds [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 2946-2963. |
| [6] | Zhiyan Ma, Yunjian Li, Xiao-Qiang Sun, Ke Yang, Zheng-Yi Li. Calixarene Promoted Transition-Metal-Catalyzed Reactions [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2188-2201. |
| [7] | Sai Wu, Wuxi Tao, Guo Wang, Bin Zhao, Huajie Chen. Synthesis and Optoelectronic Properties of A-D-A Type Small Molecule Acceptors Containing Isatin-Fused Acenaphthenequinone Imide Terminal Groups [J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 2019-2028. |
| [8] | San'e Zhu, Lifeng Dou, Jianhui Zhang, Ying Wu, Wei Yang, Hongdian Lu, Chunxiang Wei, Chonghai Deng, Qiang Dong. Palladium-Catalyzed Synthesis of Dihydrofuran-Fused [60]Fullerene Derivatives via Heteroannulation of Olefins [J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 2082-2090. |
| [9] | Yuting Liu, Jie Li, Dawei Yin. Progress of Ferrocene-Based Metal Cation Recognition Receptor [J]. Chinese Journal of Organic Chemistry, 2021, 41(1): 158-170. |
| [10] | Liu Xu-Bo, Lin Jia-Le, Wang Hui, Zhang Dan-Wei, Li Zhan-Ting. Water-Solubilization of Acyclic Cucurbiturils for Arenes and Aromatic Aldehydes and the Promotion for the Generation of Two Hydrazine-Based Macrocycles [J]. Chinese Journal of Organic Chemistry, 2020, 40(3): 663-668. |
| [11] | Li Jing, Han Ying, Chen Chuanfeng. Recent Advances in Novel Macrocyclic Arenes [J]. Chinese Journal of Organic Chemistry, 2020, 40(11): 3714-3737. |
| [12] | Niu Chuang, Wang Guanwu. Progress in Electrochemical Reactions of[60]Fullerene-Fused Heterocycles [J]. Chinese Journal of Organic Chemistry, 2020, 40(11): 3633-3645. |
| [13] | Yan Meng, Peng Wenchang, Wang Hui, Zhang Danwei, Li Zhanting. Supramolecular Organic Framework Loading for Camptothecin Open-Ring Carboxylates and Their Lactonization Kinetics [J]. Chin. J. Org. Chem., 2019, 39(9): 2567-2573. |
| [14] | Sheng Jiajun, Yu Ya'nan, Wang Xin, Qian Yu, Fu Liwu, Zhao Yun, Ma Mingliang, Hu Wenhao. Synthesis of Paclitaxel Side Chain via Multi-Component Reaction and Its Application to the Synthesis of Paclitaxel Analogues [J]. Chin. J. Org. Chem., 2019, 39(2): 377-389. |
| [15] | Sun, Weidong, Ye, Lin, Liu, Jia, Zheng, Lu, Guo, Wencai, Han, Senkai, Shao, Chengyuan, Jiang, Hua. Self-Assembled of Corannulene-Based Molecular Cage with Fullerenes as Template [J]. Chinese Journal of Organic Chemistry, 2019, 39(10): 2867-2874. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||