Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (2): 436-454.DOI: 10.6023/cjoc202208020 Previous Articles Next Articles
吴江龙†, 王中杰†, 王晨宇, 王彦, 李红俊, 罗辉, 李昊, 王富强*( ), 李典军*(
), 李典军*( ), 杨金会*(
), 杨金会*( )
)
收稿日期:2022-08-16
修回日期:2022-09-18
发布日期:2022-10-31
作者简介:基金资助:
Jianglong Wu†, Zhongjie Wang†, Chenyu Wang, Yan Wang, Hongjun Li, Hui Luo, Hao Li, Fuqiang Wang( ), Dianjun Li(
), Dianjun Li( ), Jinhui Yang(
), Jinhui Yang( )
)
Received:2022-08-16
Revised:2022-09-18
Published:2022-10-31
Contact:
*E-mail: About author:Supported by:Share
Jianglong Wu, Zhongjie Wang, Chenyu Wang, Yan Wang, Hongjun Li, Hui Luo, Hao Li, Fuqiang Wang, Dianjun Li, Jinhui Yang. Research Progress on the Synthesis of Nitrogen-Containing Compounds with Cyanamide as a Building Block[J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 436-454.
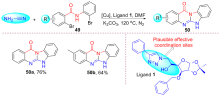
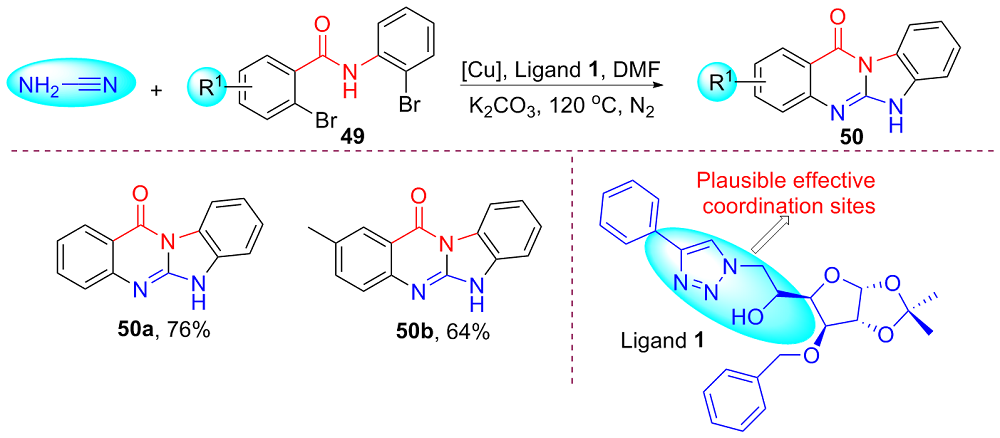
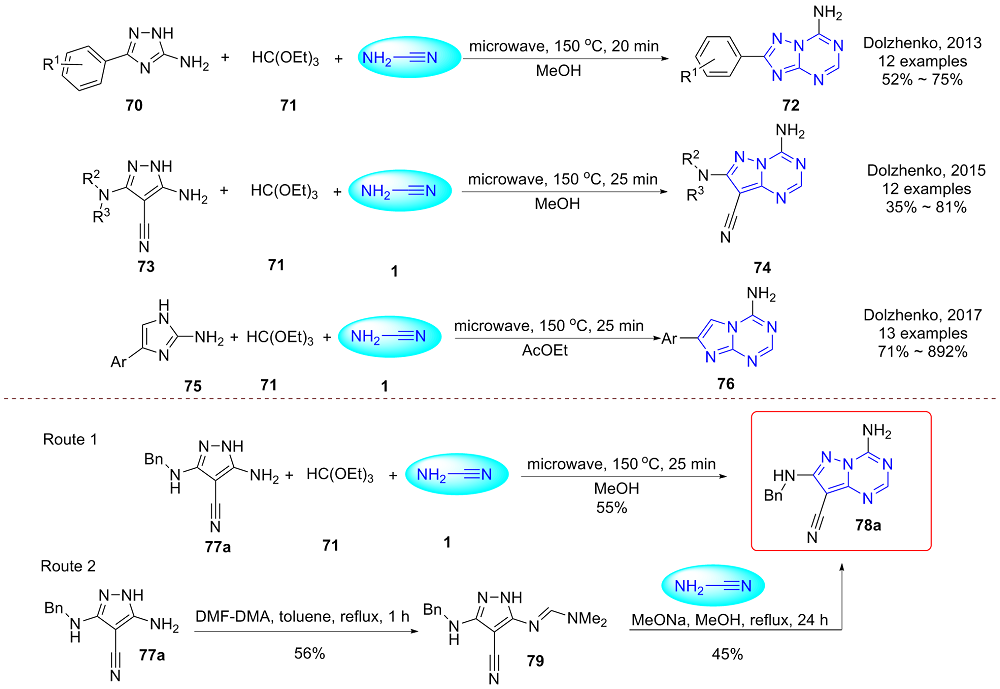
| [1] |
Romagnoli, R.; Baraldi, P. G.; Carrion, M. D. J. Med. Chem. 2009, 52, 5551.
doi: 10.1021/jm9001692 pmid: 19663386 |
| [2] |
Abdelazeem, A. H.; Abdelatef, S. A.; El-Saadi, M. T. Eur. J. Pharm. Sci. 2014, 62,197.
|
| [3] |
Zhu, Y.; Loso, M. R.; Watson, G. B. J. Agric. Food Chem. 2011, 59, 2950.
doi: 10.1021/jf102765x |
| [4] |
Lainé, D.; Palovich, M.; McCleland, B. ACS Med. Chem. Lett. 2011, 2, 142.
doi: 10.1021/ml100212k |
| [5] |
Stafford, J. A.; Feldman, P. L. Annu. Rep. Med. Chem. 1996, 31,71.
|
| [6] |
David, H. D.; Samuel, W. G.; James, A. L. Tetrahedron Lett. 1997, 38, 3377.
doi: 10.1016/S0040-4039(97)00653-9 |
| [7] |
Berlinck, R. G. S. Fortschr. Chem. Org. Naturst. 1995, 66,119.
|
| [8] |
Castagnolo, D.; Schenone, S.; Botta, M. Chem. Rev. 2011, 111, 5247.
doi: 10.1021/cr100423x pmid: 21657224 |
| [9] |
Patrick, C. K.; Monica, F.; John, A. F. Tetrahedron Lett. 1998, 39, 2663.
|
| [10] |
Kamo, T.; Endo, M.; Sato, M. Phytochemistry 2008, 69, 1166.
doi: 10.1016/j.phytochem.2007.11.004 |
| [11] |
Chen, X.; Orser, B. A.; MacDonald, J. F. Eur. J. Pharmacol. 2010, 648, 15.
doi: 10.1016/j.ejphar.2010.09.005 |
| [12] |
Ojo, B.; Dunbar, P. G.; Durant, G. J. Bioorg. Med. Chem. 1996, 4, 1605.
doi: 10.1016/0968-0896(96)00152-6 |
| [13] |
Cannon, C. P. Clin. Cardiol. 2003, 26, 358.
doi: 10.1002/clc.4950260803 |
| [14] |
Kotthaus, J.; Steinmetzer, T.; van de Locht, A. J. Enzyme Inhib. Med. Chem. 2011, 26, 115.
doi: 10.3109/14756361003733647 |
| [15] |
Sävmarker, J.; Rydfjord, J.; Gising, J.; Odell, L. R.; Larhed, M. Org. Lett. 2012, 14, 2394.
doi: 10.1021/ol300813c |
| [16] |
Rydfjord, J.; Svensson, F.; Trejos, A.; Sjçberg, J. R.; Skçld, C.; vmarker, J. S.; Odell, L. R.; Larhed, M. Chem. Small. 2013, 19, 13803.
|
| [17] |
Rydfjord, J.; Skillinghaug, B.; Brandt, P.; Odell, L. R.; Larhed, M. Org. Lett. 2017, 19, 4066.
doi: 10.1021/acs.orglett.7b01836 pmid: 28741950 |
| [18] |
Klein, M.; Güthner, T.; Sans, J. Green Chem. 2021, 23, 3289-3294.
doi: 10.1039/D1GC00700A |
| [19] |
(a) Odo, K.; Sugino, K. J. Electrochem. Soc. 1957, 104, 160.
doi: 10.1149/1.2428525 |
|
(b) Ichikawa, E.; Odo, K. Denki Kagaku 1964, 32, 897.
|
|
| [20] |
Katritzky, A. R.; Khashab, N. M.; Bobrov, S. Helv. Chim. Acta 2005, 88, 1664.
doi: 10.1002/hlca.200590131 |
| [21] |
Yong, Y. F.; Kowalski, J.; Lipton, M. J. Org. Chem. 1997, 62, 1540.
doi: 10.1021/jo962196k |
| [22] |
Andrews, D.; Finlay, M. R.; Green, C.; Jones, C.; Oza, V. WO 095159, 2006.
|
| [23] |
Bicking, J. B.; Mason, J. W.; Woltersdorf Jr, O. W. J. Med. Chem. 1965, 8, 638.
doi: 10.1021/jm00329a017 |
| [24] |
Pacha, W.; Salzmann, R.; Scholtysik, G. Br. J. British. J. Pharmacol. 1975, 53, 513.
|
| [25] |
Åkerbladh, L.; Schembri, L S.; Larhed, M.; Odell, L. R. J. Org. Chem. 2017, 82, 12520.
doi: 10.1021/acs.joc.7b02294 pmid: 29027801 |
| [26] |
Miller, A. J. Org. Chem. 1984, 49, 4072.
doi: 10.1021/jo00195a043 |
| [27] |
Mancheno, O. G.; Bistri, O.; O.; Bolm, C. Org. Lett. 2007, 9, 3809.
doi: 10.1021/ol7016577 |
| [28] |
(a) Serban, G. Molecules 2020, 25, 942.
doi: 10.3390/molecules25040942 |
|
(b) Gao, F.; Wang, T.; Xiao, J.; Huang, G. Eur. J. Med. Chem. 2019, 173, 274.
doi: 10.1016/j.ejmech.2019.04.043 |
|
| [29] |
Yin, P.; Ma, W. B.; Chen, Y.; Huang, W. C.; Deng, Y.; He, L. Org. Lett. 2009, 5482.
|
| [30] |
(a) Hiskey, M.; Chavez, D. E.; Naud, D. L. Proc. Int. Pyrotech. Semin. 2000, 27, 3.
pmid: 7031768 |
|
(b) Singh, H.; Chawla, A. S.; Kapoor, V. K. Prog. Med. Chem. 1980, 17, 151.
pmid: 7031768 |
|
| [31] |
Moderhack, D. J. Prakt. Chem. 1998, 340, 687.
doi: 10.1002/prac.19983400802 |
| [32] |
García Mancheño, O.; Bolm, C. Org. Lett. 2007, 9, 2951.
pmid: 17595098 |
| [33] |
For an example of guanidine synthesis with cyanamide and carbodiimide see: Mestres, R.; Palomo, C. Synthesis 1980, 755.
|
| [34] |
Baeten, M.; Maes, B. U. W. Adv. Synth. Catal. 2016, 358, 826.
doi: 10.1002/adsc.201501146 |
| [35] |
Nordeman, P.; Chow, S. Y.; Odell, A. F. Org. Biomol. Chem. 2017, 15, 4875.
doi: 10.1039/c7ob01064h pmid: 28537303 |
| [36] |
Ma, X. D.; Huang, W.; Yang, Y. Y. CN 112979555, 2021.
|
| [37] |
Lunn, W. H. W.; Harper, R. W.; Stone, R. L. J. Med. Chem. 1971, 14, 1069.
pmid: 5115206 |
| [38] |
(a) Li, X.; Gan, B.; Xie, T. J. Chem. Res. 2016, 40, 178.
doi: 10.3184/174751916X14558161990884 |
|
(b) Hua, G.; Li, Y.; Fuller, A. Eur. J. Org. Chem. 2009, 2009, 1612.
doi: 10.1002/ejoc.200900013 |
|
| [39] |
(a) Bird, C. W. Tetrahedron 1965, 21, 2179.
doi: 10.1016/S0040-4020(01)98354-1 pmid: 17194113 |
|
(b) Bird, C. W.; Kapilli, M. Tetrahedron 1987, 43, 4621.
doi: 10.1016/S0040-4020(01)86904-0 pmid: 17194113 |
|
|
(c) Fadda, A. A.; Refat, H. M.; Zaki, M. E.; Monir, A. E. Synth. Commun. 2001, 31, 3537.
doi: 10.1081/SCC-100106216 pmid: 17194113 |
|
|
(d) Carpenter, R. D.; Lam, K. S.; Kurth, M. J. J. Org. Chem. 2007, 72, 284.
pmid: 17194113 |
|
| [40] |
Yang, D. S.; Wang, Y. Y.; Yang, H. J.; Liu, T.; Fu, H. Adv. Synth. Catal. 2012, 354, 477.
doi: 10.1002/adsc.201100580 |
| [41] |
Lou, Z. B.; Wu, X. D.; Yang, H. J.; Zhu, C. J.; Fu, H. Adv. Synth. Catal. 2015, 357, 3961.
doi: 10.1002/adsc.201500577 |
| [42] |
Mishra, N.; Singh, A. S.; Agrahari, A. K.; Singh, S. K.; Singh, M.; Tiwari, V. K. ACS Comb. Sci. 2019, 21, 389.
doi: 10.1021/acscombsci.9b00004 pmid: 30943366 |
| [43] |
Farhanullah
pmid: 12662083 |
| [44] |
Zhang, L.; Peng, X. M.; Damu, G. L. V. Med. Res. Rev. 2014, 34, 340.
doi: 10.1002/med.21290 pmid: 23740514 |
| [45] |
Anastasi, C.; Crowe, M. A.; Powner, M. W.; Sutherland, J. D. Angew. Chem., nt. Ed. 2006, 45, 6176.
|
| [46] |
Fahrenbach, A. C.; Giurgiu, C.; Tam, C. P.; Li, L.; Hongo, Y.; Aono, M.; Szostak, J. W. J. Am. Chem. Soc. 2017, 139, 8780.
doi: 10.1021/jacs.7b01562 pmid: 28640999 |
| [47] |
Cohen, S. H.; Gerding, D. N.; Johnson, S. Infect. Control Hosp. Epidemiol. 2010, 31, 431.
doi: 10.1086/651706 |
| [48] |
Malamas, M. S.; Erdei, J.; Gunawan, I. J. Med. Chem. 2010, 53, 1146.
doi: 10.1021/jm901414e |
| [49] |
Li, W. T.; Hwang, D. R.; Song, J. S. J. Med. Chem. 2010, 53, 2409.
doi: 10.1021/jm901501s |
| [50] |
Nishimura, T.; Kitajima, K. J. Org. Chem. 1979, 44, 818.
doi: 10.1021/jo01319a034 |
| [51] |
Little, T. L.; Webber, S. E. J. Org. Chem. 1994, 59, 7299.
doi: 10.1021/jo00103a021 |
| [52] |
Molina, P.; Fresneda, P.; Sanz, M. J. Org. Chem. 1999, 64, 2540.
doi: 10.1021/jo9819382 |
| [53] |
Liu, S.; Shao, J.; Guo, X.; Luo, J.; Zhao, M.; Zhang, G. L.; Yu, Y. P. Tetrahedron 2014, 70, 1418.
doi: 10.1016/j.tet.2014.01.007 |
| [54] |
Guo, X.; Chen, W. T.; Chen, B. H.; Huang, W.; Qi, W. X.; Zhang, G. L.; Yu, Y. P. Org. Lett. 2015, 17, 1157.
doi: 10.1021/acs.orglett.5b00289 |
| [55] |
Burnstock, G.; Verkhratsky, A. Acta. Physiol. 2009, 195, 415.
doi: 10.1111/j.1748-1716.2009.01957.x |
| [56] |
Murray, J. M.; Bussiere, D. E. Chemogenomics 2009, 575, 47.
|
| [57] |
Li, X.; Kang, H.; Liu, X.; Liu, Z.; Shu, K.; Chen, X.; Zhu, S. J. Huazhong Univ. Sci. Technol. Med. Sci. 2012, 32, 257.
|
| [58] |
Gillespie, R. J.; Bamford, S. J.; Botting, R. J. Med. Chem. 2009, 52, 33.
doi: 10.1021/jm800961g pmid: 19072055 |
| [59] |
http://clinicaltrials.gov/ct2/show/NCT00442780
|
| [60] |
Kalinina, S. A.; Kalinin, D. V.; Dolzhenko, A. V. Tetrahedron Lett. 2013, 54, 5537.
doi: 10.1016/j.tetlet.2013.07.158 |
| [61] |
Lim, F. P. L.; Luna, G; Dolzhenko, A. V. Tetrahedron Lett. 2015, 56, 7016.
doi: 10.1016/j.tetlet.2015.11.006 |
| [62] |
Kalinin, D.; Kalinina, S.; Dolzhenko, A. Heterocycles 2013, 87, 147.
doi: 10.3987/COM-12-12601 |
| [63] |
Lim, F. P. L.; Low, S. T.; Ho, E. L. K.; Halcovitch, N. R.; Tiekinkc, E. R. T.; Dolzhenko, A. V. RSC Adv. 2017, 7, 51062.
doi: 10.1039/C7RA11305F |
| [64] |
Li, T.; Senesi, A. J.; Lee, B. Chem. Rev. 2016, 116, 80.
doi: 10.1021/acs.chemrev.5b00483 |
| [65] |
Mahfoudh, M.; Abderrahim, R.; Leclerc, E. Eur. J. Org. Chem. 2017, 2017, 2856.
doi: 10.1002/ejoc.201700008 |
| [66] |
Zhang, L.; Fan, J.; Chong, J. H. Bioorg. Med. Chem. Lett. 2011, 21, 5633.
doi: 10.1016/j.bmcl.2011.06.129 pmid: 21798738 |
| [67] |
Lim, S. M.; Xie, T.; Westover, K. D. Bioorg. Med. Chem. Lett. 2015, 25, 3382.
doi: 10.1016/j.bmcl.2015.04.103 |
| [68] |
Venkatesan, G.; Paira, P.; Cheong, S. L. Bioorg. Med. Chem. Lett. 2014, 22, 1751.
doi: 10.1016/j.bmc.2014.01.018 |
| [69] |
(a) Woller, K. R.; Curtin, M. L.; Frank, K. E.; Josephsohn, N. S.; Li, B. C.; Wishart, N. WO 156698, 2011.
|
|
(b) Wainwright, P.; Maddaford, A.; Simms, M. Synlett 2011, 1900.
|
|
| [70] |
Tsai, S. E.; Yen, W. P.; Tseng, C. C.; Xie, J. J.; Liou, M. Y.; Li, Y. T.; Uramaru, N.; Wong, F. F. Tetrahedron 2018, 74, 2787.
doi: 10.1016/j.tet.2018.04.048 |
| [71] |
Tseng, C. C.; Tsai S. E.; Li S. M. Wong, F. F. J. Org. Chem. 2019, 84, 16157.
doi: 10.1021/acs.joc.9b02653 |
| [72] |
Seela, F.; Steker, H. Heterocycles 1985, 23, 2521.
doi: 10.3987/R-1985-10-2521 |
| [73] |
Su, L.; Sun, K.; Pan, N.; Liu, L.; Sun, M. L.; Dong, J. Y.; Zhou, Y. B. Org. Lett. 2018, 20, 3399.
doi: 10.1021/acs.orglett.8b01324 |
| [74] |
(a) Qiao, Q.; Dominique, R.; Goodnow, Jr. R. Tetrahedron Lett. 2008, 49, 3682.
doi: 10.1016/j.tetlet.2008.03.140 |
|
(b) Desroy, N.; Moreau, F.; Briet, S. Bioorg. Med. Chem. 2009, 17, 1276.
doi: 10.1016/j.bmc.2008.12.021 |
|
| [75] |
Bey, E.; Marchais-Oberwinkler, S.; Werth, R. J. Med. Chem. 2008, 51, 6725.
doi: 10.1021/jm8006917 |
| [76] |
Chalopin, T.; Dorta, D. A.; Sivignon, A. Org. Biomol Chem. 2016, 14, 3913.
doi: 10.1039/c6ob00424e pmid: 27043998 |
| [77] |
Ishita, K.; Stefanopoulos, S.; Khalil, A. Bioorg. Med. Chem. 2018, 26, 2251.
|
| [78] |
Nettekoven, M.; Guba W.; Neidhart, W. ChemMedChem 2006, 1, 45.
pmid: 16892333 |
| [79] |
Fu, R. G.; Wang, Y.; Xia, F. J. Org. Chem. 2019, 84, 12237.
doi: 10.1021/acs.joc.9b02032 |
| [80] |
(a) Luo, L.; Meng, L.; Sun, Q. Tetrahedron Lett. 2014, 55, 259.
doi: 10.1016/j.tetlet.2013.11.014 |
|
(b) Gruner, M.; Boettcher, G.; Gewald, K. J. Heterocycl. Chem. 2008, 45, 1071.
doi: 10.1002/jhet.5570450419 |
|
| [81] |
(a) Kumar, S. V.; Parameshwarappa, G.; Ila, H. J. Org. Chem. 2013, 78, 7362.
doi: 10.1021/jo401208u pmid: 11950363 |
|
(b) Hodgetts, K. J.; Kershaw, M. T. Org. Lett. 2003, 5, 2911.
pmid: 11950363 |
|
|
(c) Hodgetts, K. J.; Kershaw, M. T. Org. Lett. 2002, 4, 1363.
pmid: 11950363 |
|
| [82] |
Guo, S.; Zhao, D.; Zhu, Y. Synth. Commun. 2017, 47, 1758.
doi: 10.1080/00397911.2017.1350275 |
| [83] |
Avadhani, A.; Iniyavan, P.; Kumar, Y.; Ila, H. J. Org. Chem. 2021, 86, 8508.
doi: 10.1021/acs.joc.1c00616 pmid: 34107686 |
| [84] |
(a) Romagnoli, R.; Baraldi, P. G.; Salvador, M. K. J. Med. Chem. 2012, 55, 5433.
doi: 10.1021/jm300388h pmid: 22027100 |
|
(b) Romagnoli, R.; Baraldi, P. G.; Cara, C. L. Eur. J. Med. Chem. 2011, 46, 6015.
doi: 10.1016/j.ejmech.2011.10.013 pmid: 22027100 |
|
| [85] |
Kathiravan, M. K.; Salake, A. B.; Chothe, A. S. Bioorg. Med. Chem. 2012, 20, 5678.
doi: 10.1016/j.bmc.2012.04.045 |
| [86] |
Mahfoudh, M.; Abderrahim, R.; Leclerc, E. Eur. J. Org. Chem. 2017, 2017, 2856.
doi: 10.1002/ejoc.201700008 |
| [87] |
Chaurasia, S. R.; Dange, R.; Bhanage, B. M. Catal. Commun. 2020, 137, 105933.
doi: 10.1016/j.catcom.2020.105933 |
| [88] |
Wolf, F. J.; Pfister 3rd. K.; Wilson, Jr, R. M.; Robinson, C. A. J. Am. Chem. Soc. 1954, 76, 3551.
doi: 10.1021/ja01642a060 |
| [89] |
Belsky, A. J.; Li, T. J.; Brill, T. B. J. Supercrit. Fluids 1997, 10, 201.
doi: 10.1016/S0896-8446(97)00022-3 |
| [90] |
Zhao, P.; Zhou, Y.; Yu, X. X. Huang, C.; Wu, Y. D.; Yin, G. D. Org. Lett. 2020, 22, 8528.
doi: 10.1021/acs.orglett.0c03130 |
| [91] |
(a) Wu, C. P.; Lusvarghi, S.; Wang, J. C. Mol. Pharmaceutics 2019, 16, 3040.
doi: 10.1021/acs.molpharmaceut.9b00274 pmid: 30807645 |
|
(b) Grünewald, S.; Politz, O.; Bender, S. Int. J. Cancer 2019, 145, 1346.
doi: 10.1002/ijc.32224 pmid: 30807645 |
|
|
(c) Rouge, T. L. M.; Galluzzi, L.; Olaussen, K. A. Cancer Res. 2007, 67, 6253.
doi: 10.1158/0008-5472.CAN-07-0538 pmid: 30807645 |
|
| [92] |
(a) Dixon, J. A.; Phillips, B.; Achebe, F.; Kluender, H. C. E.; Newcom, J.; Parcella, K.; Magnuson, S.; Hong, Z.; Zhang, Z.; Liu, Z.; Khire, U.; Wang, L.; Michels, M.; Chandler, B.; ,O’Connor S. US 8143393, 2006.
pmid: 28851503 |
|
(b) O'Connor, S.; Dumas, J.; Lee, W.; Dixon, J.; Cantin, D.; Gunn, D.; Burke, J.; Phillips, B.; Lowe, D.; Shelekhin, T.; Wang, G.; Ma, X.; Ying, S.; McClure, A.; Achebe, F.; Lobell, M.; Ehrgott, F.; Iwuagwu, C.; Parcella, K. US 8431695, 2006.
pmid: 28851503 |
|
|
(c) Song, Y.; Zhan, P.; Zhang, Q.; Liu, X. Curr. Pharm. Des. 2013, 19, 1528.
pmid: 28851503 |
|
|
(d) Cascioferro, S.; Parrino, B.; Spano, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G. Eur. J. Med. Chem. 2017, 142, 328.
doi: S0223-5234(17)30612-8 pmid: 28851503 |
|
| [93] |
Paymode, D. J.; Cardoso, F. S. P.; Agrawal, T.; Tomlin, J. W.; Cook, D. W.; Burns, J. M.; Stringham, R. W.; Sieber, J. D.; Snead, D. R. Org. Lett. 2020, 22, 7656.
doi: 10.1021/acs.orglett.0c02848 pmid: 32931286 |
| [94] |
Knapp, R. R.; Tona, V.; Okada, T.; Sarpong, R.; Garg, N. K. Org. Lett. 2020, 22, 8430.
doi: 10.1021/acs.orglett.0c03052 |
| [95] |
Wei, Z.; Zhang, Q.; Tang, M. Org. Lett. 2021, 23, 4436.
doi: 10.1021/acs.orglett.1c01379 |
| [96] |
Liu, X.; Liu, H.; Bian, C.; Hu, Y. L. J. Org. Chem. 2022, 87, 5882.
doi: 10.1021/acs.joc.2c00176 |
| [1] | Kangbo Feng, Jiong Chen, Shuangxi Gu, Haifeng Wang, Fen'er Chen. New Progress of Fully Continuous Flow Reaction Technologies in Pharmaceutical Synthesis (2019~2022) [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 378-397. |
| [2] | Chuanchuan Wang, Zhiwei Ma, Xuehui Hou, Longhua Yang, Yajing Chen. Research and Application of N-Ts Cyanamides in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 74-93. |
| [3] | Xinyi Ren, Guangzhu Wang, Xiaolei Ji, Kaiwu Dong. Synthesis of Two Types of Nitriles Both Bearing Quaternary Carbon Centers in One-Pot Manner [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 526-533. |
| [4] | Xiaojing Tian, Zhenzhen Fan, Si Jiang, Zhiwei Li, Jiangsheng Li, Yuefei Zhang, Cuihong Lu, Weidong Liu. Metal-Free Synthesis of Benzimidazo[1,2-c]quinazolines from N-Cyanobenzimidazoles via Double C—H Functionalizations [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3684-3692. |
| [5] | Yuan Zhu, Leyuan Chen, Wenbin Hou, Yiliang Li. Recent Progress in Nucleophilic Fluoride Mediated Fluorine-18 Labeling of Arenes and Heteroarenes [J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 1774-1788. |
| [6] | Jiantao Zhang, Peng Zhou, Duoduo Xiao, Weibing Liu. Research Progress of 1,3,5-Triazinanes in the Synthesis of Nitrogen-Containing Heterocycles [J]. Chinese Journal of Organic Chemistry, 2021, 41(11): 4154-4166. |
| [7] | Li Jihui, Li Zhengzhang, Zhang Yucang, Xu Wenrong, Xu Shuying. Progress on the Synthesis and Applications of Cyanamides [J]. Chin. J. Org. Chem., 2017, 37(8): 1903-1915. |
| [8] | Lu Wenyu, Yu Wenjing, Sun Dequn. Advances in Application of Marine Bioactive Peptides in Drug Development [J]. Chin. J. Org. Chem., 2017, 37(7): 1681-1700. |
| [9] | Huang Yanmin, Yao Qiucui, Liu Zhiping, Gan Chunfang, Zheng Jiahua, Sheng Haibing, Shi Haishing, Cui Jianguo. Synthesis and Antiproliferative Evaluation of Chenodeoxycholic Acid Containing-Nitrogen Derivatives [J]. Chin. J. Org. Chem., 2015, 35(10): 2168-2175. |
| [10] | SHU Long-Yi, SUN Hu, WANG Qian, TUN Shi. Progress in Binding Affinities of Metal Porphyrins to Heterocycles and DNA [J]. Chin. J. Org. Chem., 2009, 29(11): 1700-1707. |
| [11] | Li, Yunbo ; Tang, Fengxiang*; Meng, Chun; Guo, Yanghao. Progress in the Synthesis and Application of Nipecotic Acid and Its Derivatives [J]. Chin. J. Org. Chem., 2009, 29(07): 1068-1081. |
| [12] | WEI, Rong-Bao* ; LIU, Bo; LIU, Yang; GUO, Jin-Jing; ZHANG, Da-Wei. Progress in the Studies on Biological Activities of O, N or S-Containing Spiro Compounds [J]. Chin. J. Org. Chem., 2008, 28(9): 1501-1514. |
| [13] | LI,Lu; ZHANG, Gui-Sheng*. Progress in Syntheses of Uncommon Sugars [J]. Chin. J. Org. Chem., 2008, 28(07): 1129-1137. |
| [14] | He Hongwu. Phosphorus chemistry,A field full of vitality and scientific opportunities [J]. Chin. J. Org. Chem., 2003, 23(2): 155-161. |
| [15] | Liu Liu;Zhang Haitao;Wang Yunpu. Syntheses of water-soluble polymer-supported ferrocenyl schiff bases [J]. Chin. J. Org. Chem., 2002, 22(4): 286-288. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||