-
-
 About the Cover:The recent research progress in the synthesis of organo-silicons by copper-catalyzed silyl addition of silyl rea-gents to diverse unsaturated compounds such as α,β- unsaturated carbonyl compounds, imines, Morita-Baylis- Hillman (MBH) alcohols, p-quinone methides, α,β-unsa- turated alkyne carbonyl compounds and α,β-unsaturated sulfones is summarized by Han, Li, Chen, Zhang, Zhao, Zhang, and Zhu on page 555. It will largely promote the development of such copper-catalyzed silyl addition reactions.
About the Cover:The recent research progress in the synthesis of organo-silicons by copper-catalyzed silyl addition of silyl rea-gents to diverse unsaturated compounds such as α,β- unsaturated carbonyl compounds, imines, Morita-Baylis- Hillman (MBH) alcohols, p-quinone methides, α,β-unsa- turated alkyne carbonyl compounds and α,β-unsaturated sulfones is summarized by Han, Li, Chen, Zhang, Zhao, Zhang, and Zhu on page 555. It will largely promote the development of such copper-catalyzed silyl addition reactions. -
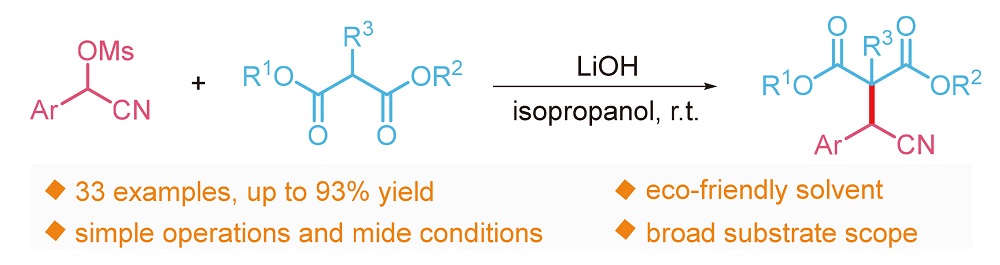
 About the Cover:The synthesis of α-aryl nitriles via nucleophilic sub- stitution of α-cyanohydrin methanesulfonates with malo- nates is reported by Wang, Zheng, Zhou, Wang, Yan, Wang and Chen on page 668. This transition metal-free protocol has the advantages of cheap and easily avai- lable starting materials, mild reaction conditions, simple operation, a broad substrate scope and high functional group tolerance. Furthermore, this strategy could also be used to asymmetric malonates and acyl esters.
About the Cover:The synthesis of α-aryl nitriles via nucleophilic sub- stitution of α-cyanohydrin methanesulfonates with malo- nates is reported by Wang, Zheng, Zhou, Wang, Yan, Wang and Chen on page 668. This transition metal-free protocol has the advantages of cheap and easily avai- lable starting materials, mild reaction conditions, simple operation, a broad substrate scope and high functional group tolerance. Furthermore, this strategy could also be used to asymmetric malonates and acyl esters. -
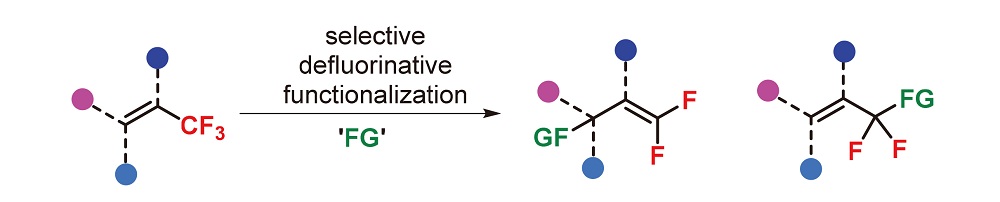
 About the Cover:The recent progress in selective C—F bond activation of trifluoromethyl akenes is reviewed by Xu and Song on page 411. The research progress in this area is summa-rized, including nucleophilic addition/defluorination (SN2' type), acid-promoted defluorinative Friedel-Crafts arylation, (formal) ipso-defluorinative functionalization, and transition-metal-catalyzed migratory insertion/de- fluorination. Finally, the future direction in this area is also prospected.
About the Cover:The recent progress in selective C—F bond activation of trifluoromethyl akenes is reviewed by Xu and Song on page 411. The research progress in this area is summa-rized, including nucleophilic addition/defluorination (SN2' type), acid-promoted defluorinative Friedel-Crafts arylation, (formal) ipso-defluorinative functionalization, and transition-metal-catalyzed migratory insertion/de- fluorination. Finally, the future direction in this area is also prospected. -
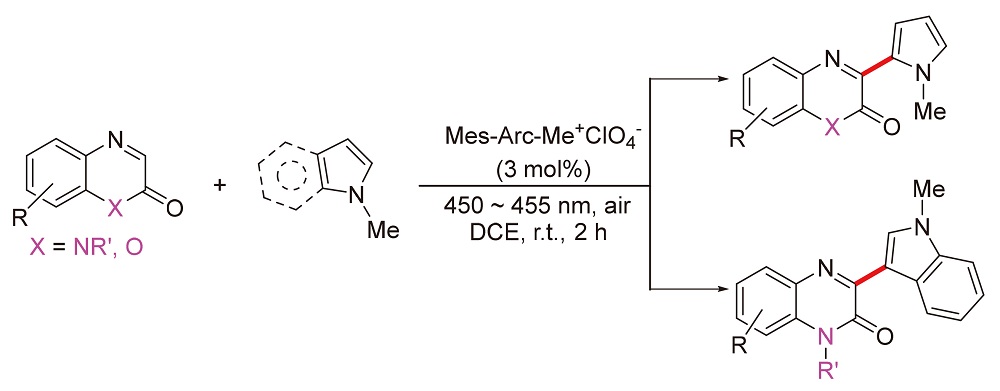
 About the Cover:One feasible methodology has been developed for oxi-dative cross-coupling between quinoxalinones and pyr-roles. Mechanism studies show that electron-rich pyrroles own nearly ten times quenching rate constant than that of electron-deficient quinoxalinones. Utilizing the commerial available photocatalyst Mes-Acr-Me+ClO-4(3 mol%), the oxidative coupling products of quinolones and pyrrole derivatives have been synthesized by Shen, Li, Zhou, Wang and Wang on page 697.
About the Cover:One feasible methodology has been developed for oxi-dative cross-coupling between quinoxalinones and pyr-roles. Mechanism studies show that electron-rich pyrroles own nearly ten times quenching rate constant than that of electron-deficient quinoxalinones. Utilizing the commerial available photocatalyst Mes-Acr-Me+ClO-4(3 mol%), the oxidative coupling products of quinolones and pyrrole derivatives have been synthesized by Shen, Li, Zhou, Wang and Wang on page 697.
-
-
Current Issue