Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (8): 2790-2799.DOI: 10.6023/cjoc202302012 Previous Articles Next Articles
刘敏a,†, 杨冬燕b,†, 肖玉梅a,*( ), 苏旺苍c, 赵峰海a, 覃兆海a,*(
), 苏旺苍c, 赵峰海a, 覃兆海a,*( )
)
收稿日期:2023-02-12
修回日期:2023-04-04
发布日期:2023-06-26
作者简介:基金资助:
Min Liua,†, Dongyan Yangb,†, Yumei Xiaoa( ), Wangcang Suc, Fenghai Zhaoa, Qin Zhaohai .a(
), Wangcang Suc, Fenghai Zhaoa, Qin Zhaohai .a( )
)
Received:2023-02-12
Revised:2023-04-04
Published:2023-06-26
Contact:
*E-mail: About author:Supported by:Share
Min Liu, Dongyan Yang, Yumei Xiao, Wangcang Su, Fenghai Zhao, Qin Zhaohai .. Synthesis and Bioactivities of 5-Nitroimino-[1,4-2H]-1,2,4-triazolines as Olefin-Imidacloprid Mimics[J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2790-2799.
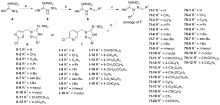

| Compd. | Honey bee toxicity | Compound | Honey bee toxicity | Compd. | Honey bee toxicity |
|---|---|---|---|---|---|
| Ⅰ-3 | Low | Ⅱ-2 | Low | 7Ⅰ-19 | Low |
| Ⅰ-7 | Low | Ⅱ-5 | Low | 7Ⅰ-20 | Low |
| Ⅰ-9 | Low | Ⅱ-7 | Low | 3 | High[ |
| Ⅰ-13 | Low | Ⅱ-10 | Low | ||
| Ⅰ-18 | Low | Ⅱ-12 | Low |
| Compd. | Honey bee toxicity | Compound | Honey bee toxicity | Compd. | Honey bee toxicity |
|---|---|---|---|---|---|
| Ⅰ-3 | Low | Ⅱ-2 | Low | 7Ⅰ-19 | Low |
| Ⅰ-7 | Low | Ⅱ-5 | Low | 7Ⅰ-20 | Low |
| Ⅰ-9 | Low | Ⅱ-7 | Low | 3 | High[ |
| Ⅰ-13 | Low | Ⅱ-10 | Low | ||
| Ⅰ-18 | Low | Ⅱ-12 | Low |
| Compd. | R2 | R1 | A.craccivora/(μg•mL-1) | N. lugens/(μg•mL-1) | F. occidentalis/(μg•mL-1) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 400 | 100 | 400 | 100 | 400 | 100 | 50 | |||||||||||||||
| Ⅰ-1 | | H | 0 | — | 0 | — | 6.67 | — | — | ||||||||||||
| Ⅰ-2 | CH3 | 44 | — | 4.44 | — | 0 | — | — | |||||||||||||
| Ⅰ-3 | C2H5 | 52.87 | — | 2.13 | — | 39.33 | — | — | |||||||||||||
| Ⅰ-4 | n-Pr | 41.77 | — | 4.35 | — | 8.33 | — | — | |||||||||||||
| Ⅰ-5 | i-Pr | 42.35 | — | 0 | — | 3.96 | — | — | |||||||||||||
| Ⅰ-6 | n-Bu | 26.25 | — | 0 | — | 91.09 | 0 | — | |||||||||||||
| Ⅰ-7 | sec-Bu | 22.62 | — | 0 | — | 2.27 | — | — | |||||||||||||
| Ⅰ-8 | t-Bu | 44.71 | — | 0 | — | 43.37 | — | — | |||||||||||||
| Ⅰ-9 | n-Hexyl | 62.9 | — | 6.25 | — | 3.88 | — | — | |||||||||||||
| Ⅰ-10 | n-Octyl | 0 | — | 2.13 | — | 9.09 | — | — | |||||||||||||
| Ⅰ-11 | | 2.47 | — | 4.55 | — | 22.33 | — | — | |||||||||||||
| Ⅰ-12 | 4-ClC6H4 | 3.8 | — | 0 | — | 4.3 | — | — | |||||||||||||
| Ⅰ-13 | 4-CH3C6H4 | 5.43 | — | 13.64 | — | 54.26 | — | — | |||||||||||||
| Ⅰ-14 | 4-CH3OC6H4 | 0 | — | 0 | — | 64 | — | — | |||||||||||||
| Ⅰ-15 | 4-(CH3)3CC6H4 | 0 | — | 0 | — | 4.3 | — | — | |||||||||||||
| Ⅰ-16 | C6H5 | 2.86 | — | 0 | — | 94.51 | 0 | — | |||||||||||||
| Ⅰ-17 | 3-NO2C6H4 | 9.86 | — | 8.89 | — | 2.13 | — | — | |||||||||||||
| Ⅰ-18 | 4-CNC6H4 | 3.03 | — | 0 | — | 4.4 | — | — | |||||||||||||
| Ⅱ-1 | | H | 0 | — | 0 | — | 0 | — | — | ||||||||||||
| Ⅱ-2 | CH3 | 86.25 | 0 | 0 | — | 10.42 | — | — | |||||||||||||
| Ⅱ-3 | C2H5 | 58.02 | — | 2.17 | — | 45.26 | — | — | |||||||||||||
| Ⅱ-4 | n-Pr | 52.78 | — | 0 | — | 3.49 | — | — | |||||||||||||
| Ⅱ-5 | i-Pr | 4.11 | — | 0 | — | 25.53 | — | — | |||||||||||||
| Ⅱ-6 | n-Bu | 24.29 | — | 0 | — | 3.26 | — | — | |||||||||||||
| Ⅱ-7 | sec-Bu | 100 | 0 | 6.38 | — | 100 | 100 | 0 | |||||||||||||
| Ⅱ-8 | t-Bu | 28.79 | — | 0 | — | 80.23 | 0 | — | |||||||||||||
| Ⅱ-9 | n-Hexyl | 3.49 | — | 0 | — | 28.05 | — | — | |||||||||||||
| Ⅱ-10 | n-Octyl | 42.68 | — | 2.22 | — | 2.97 | — | — | |||||||||||||
| Ⅱ-11 | | 4.84 | — | 13.64 | — | 4.3 | — | — | |||||||||||||
| Ⅱ-12 | 4-ClC6H4 | 2.44 | — | 2.17 | — | 0 | — | — | |||||||||||||
| 7I-19 | — | — | 48.48 | — | 0 | — | 4.44 | — | — | ||||||||||||
| 7I-20 | — | — | 2.41 | — | 46.51 | — | 0 | — | — | ||||||||||||
| 3 | — | — | 100 | 100 | 97.78 | 41.3 | 100 | 100 | 100 | ||||||||||||
| Nitenpyram | — | — | 100 | — | — | — | — | — | — | ||||||||||||
| Dinotefuran | — | — | — | 100 | 100 | 100 | — | — | 97.56 | ||||||||||||
| Spinosad | — | — | — | — | — | — | 100 | 100 | — | ||||||||||||
| DMF | — | — | 4.76 | 1.06 | 0 | 0 | 0 | 4.9 | 2.35 | ||||||||||||
| Compd. | R2 | R1 | A.craccivora/(μg•mL-1) | N. lugens/(μg•mL-1) | F. occidentalis/(μg•mL-1) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 400 | 100 | 400 | 100 | 400 | 100 | 50 | |||||||||||||||
| Ⅰ-1 | | H | 0 | — | 0 | — | 6.67 | — | — | ||||||||||||
| Ⅰ-2 | CH3 | 44 | — | 4.44 | — | 0 | — | — | |||||||||||||
| Ⅰ-3 | C2H5 | 52.87 | — | 2.13 | — | 39.33 | — | — | |||||||||||||
| Ⅰ-4 | n-Pr | 41.77 | — | 4.35 | — | 8.33 | — | — | |||||||||||||
| Ⅰ-5 | i-Pr | 42.35 | — | 0 | — | 3.96 | — | — | |||||||||||||
| Ⅰ-6 | n-Bu | 26.25 | — | 0 | — | 91.09 | 0 | — | |||||||||||||
| Ⅰ-7 | sec-Bu | 22.62 | — | 0 | — | 2.27 | — | — | |||||||||||||
| Ⅰ-8 | t-Bu | 44.71 | — | 0 | — | 43.37 | — | — | |||||||||||||
| Ⅰ-9 | n-Hexyl | 62.9 | — | 6.25 | — | 3.88 | — | — | |||||||||||||
| Ⅰ-10 | n-Octyl | 0 | — | 2.13 | — | 9.09 | — | — | |||||||||||||
| Ⅰ-11 | | 2.47 | — | 4.55 | — | 22.33 | — | — | |||||||||||||
| Ⅰ-12 | 4-ClC6H4 | 3.8 | — | 0 | — | 4.3 | — | — | |||||||||||||
| Ⅰ-13 | 4-CH3C6H4 | 5.43 | — | 13.64 | — | 54.26 | — | — | |||||||||||||
| Ⅰ-14 | 4-CH3OC6H4 | 0 | — | 0 | — | 64 | — | — | |||||||||||||
| Ⅰ-15 | 4-(CH3)3CC6H4 | 0 | — | 0 | — | 4.3 | — | — | |||||||||||||
| Ⅰ-16 | C6H5 | 2.86 | — | 0 | — | 94.51 | 0 | — | |||||||||||||
| Ⅰ-17 | 3-NO2C6H4 | 9.86 | — | 8.89 | — | 2.13 | — | — | |||||||||||||
| Ⅰ-18 | 4-CNC6H4 | 3.03 | — | 0 | — | 4.4 | — | — | |||||||||||||
| Ⅱ-1 | | H | 0 | — | 0 | — | 0 | — | — | ||||||||||||
| Ⅱ-2 | CH3 | 86.25 | 0 | 0 | — | 10.42 | — | — | |||||||||||||
| Ⅱ-3 | C2H5 | 58.02 | — | 2.17 | — | 45.26 | — | — | |||||||||||||
| Ⅱ-4 | n-Pr | 52.78 | — | 0 | — | 3.49 | — | — | |||||||||||||
| Ⅱ-5 | i-Pr | 4.11 | — | 0 | — | 25.53 | — | — | |||||||||||||
| Ⅱ-6 | n-Bu | 24.29 | — | 0 | — | 3.26 | — | — | |||||||||||||
| Ⅱ-7 | sec-Bu | 100 | 0 | 6.38 | — | 100 | 100 | 0 | |||||||||||||
| Ⅱ-8 | t-Bu | 28.79 | — | 0 | — | 80.23 | 0 | — | |||||||||||||
| Ⅱ-9 | n-Hexyl | 3.49 | — | 0 | — | 28.05 | — | — | |||||||||||||
| Ⅱ-10 | n-Octyl | 42.68 | — | 2.22 | — | 2.97 | — | — | |||||||||||||
| Ⅱ-11 | | 4.84 | — | 13.64 | — | 4.3 | — | — | |||||||||||||
| Ⅱ-12 | 4-ClC6H4 | 2.44 | — | 2.17 | — | 0 | — | — | |||||||||||||
| 7I-19 | — | — | 48.48 | — | 0 | — | 4.44 | — | — | ||||||||||||
| 7I-20 | — | — | 2.41 | — | 46.51 | — | 0 | — | — | ||||||||||||
| 3 | — | — | 100 | 100 | 97.78 | 41.3 | 100 | 100 | 100 | ||||||||||||
| Nitenpyram | — | — | 100 | — | — | — | — | — | — | ||||||||||||
| Dinotefuran | — | — | — | 100 | 100 | 100 | — | — | 97.56 | ||||||||||||
| Spinosad | — | — | — | — | — | — | 100 | 100 | — | ||||||||||||
| DMF | — | — | 4.76 | 1.06 | 0 | 0 | 0 | 4.9 | 2.35 | ||||||||||||
| Compd. | Inhibition rate/% (50 μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PA | BC | RS | PC | CG | FG | MG | FO | FM | |
| Ⅰ-1 | 0.0±0.8 | 14.5±3.7 | 30.0±2.7 | 6.6±0.8 | 8.0±4.6 | 1.0±2.0 | 11.1±1.0 | 0.1±2.4 | 5.1±0.2 |
| Ⅰ-2 | 0.0±1.8 | 11.2±1.6 | 20.5±2.0 | 0.6±0.8 | 5.9±3.1 | 12.1±4.1 | 6.8±1.4 | 1.0±0.5 | 2.4±1.4 |
| Ⅰ-3 | 3.6±0.4 | 11.3±3.2 | 16.9±1.6 | 3.4±1.1 | 0.0±0.4 | 14.9±0.7 | 1.6±0.7 | 0.3±0.9 | 0.2±3.9 |
| Ⅰ-4 | 0.0±0.9 | 13.3±1.1 | 11.6±3.7 | 0.0±1.4 | 11.8±0.8 | 13.6±8.8 | 4.1±0.3 | 2.3±4.2 | 1.3±3.3 |
| Ⅰ-5 | 3.2±1.8 | 6.5±1.3 | 7.6±4.3 | 8.3±0.2 | 5.8±1.1 | 0.7±3.6 | 5.6±0.4 | 3.7±3.5 | 4.2±1.4 |
| Ⅰ-6 | 4.8±0.8 | 10.7±2.8 | 14.0±1.6 | 15.5±1.1 | 13.3±0.6 | 0.0±1.2 | 5.4±1.2 | 0.1±0.3 | 2.4±1.6 |
| Ⅰ-7 | 5.4±0.4 | 13.8±1.4 | 13.3±2.4 | 20.8±0.7 | 10.9±0.3 | 0.9±0.4 | 4.1±0.7 | 0.2±0.7 | 4.2±1.7 |
| Ⅰ-8 | 12.3±2.7 | 14.8±1.0 | 10.4±4.6 | 11.5±1.6 | 6.1±3.1 | 0.0±0.2 | 6.2±1.2 | 5.4±1.4 | 6.8±4.9 |
| Ⅰ-9 | 68.2±1.0 | 35.5±0.9 | 34.1±4.7 | 50.8±2.1 | 26.4±0.9 | 12.1±1.2 | 7.0±2.2 | 21.0±1.4 | 13.5±1.5 |
| Ⅰ-10 | 89.4±0.1 | 25.2±3.0 | 33.1±2.2 | 48.7±2.4 | 18.2±3.8 | 9.2±1.3 | 20.2±0.5 | 4.9±2.7 | 54.9±1.9 |
| Ⅰ-11 | 0.0±3.5 | 8.4±4.7 | 15.9±4.2 | 1.7±1.4 | 7.1±2.3 | 0.0±2.5 | 5.4±0.7 | 4.6±1.4 | 7.437±23 |
| Ⅰ-12 | 69.7±0.9 | 20.4±1.3 | 31.8±3.5 | 10.5±3.4 | 0.0±1.4 | 9.2±3.9 | 6.1±3.4 | 13.3±1.9 | 42.1±1.0 |
| Ⅰ-13 | 75.8±1.1 | 26.5±1.5 | 23.0±1.6 | 25.3±0.9 | 11.5±2.2 | 19.0±2.5 | 7.5±2.8 | 15.3±1.8 | 47.9±3.3 |
| Ⅰ-14 | 45.7±3.9 | 16.7±2.9 | 19.3±1.7 | 14.8±2.2 | 0.0±1.4 | 17.7±1.5 | 5.8±0.7 | 8.7±2.7 | 32.2±2.5 |
| Ⅰ-15 | 87.7±0.5 | 61.2±3.4 | 59.3±2.3 | 44.0±1.2 | 21.2±4.2 | 17.2±0.5 | 10.7±3.1 | 22.7±2.3 | 59.0±0.7 |
| Ⅰ-16 | 0.0±0.8 | 15.5±3.5 | 21.0±1.9 | 6.3±0.7 | 3.7±0.9 | 8.8±2.1 | 10.4±1.2 | 5.4±0.4 | 37.6±1.6 |
| Ⅰ-17 | 0.0±2.7 | 11.6±0.9 | 21.2±1.1 | 5.3±0.6 | 10.1±2.5 | 1.5±1.2 | 2.5±1.0 | 10.3±2.6 | 6.3±0.3 |
| Ⅰ-18 | 0.0±2.2 | 10.8±2.6 | 23.9±3.7 | 0.0±0.7 | 4.7±4.6 | 9.5±2.1 | 4.9±1.8 | 1.5±1.7 | 36.1±4.5 |
| Ⅱ-1 | 0.0±1.5 | 10.4±1.7 | 5.1±1.9 | 2.4±3.2 | 15.5±0.8 | 0.4±0.7 | 5.8±0.4 | 10.6±1.4 | 3.8±1.8 |
| Ⅱ-2 | 2.0±2.0 | 6.1±4.7 | 12.4±3.4 | 0.9±0.7 | 16.8±0.4 | 4.1±2.4 | 3.3±0.7 | 4.5±3.8 | 1.5±2.2 |
| Ⅱ-3 | 0.0±2.0 | 8.6±2.9 | 21.2±4.8 | 0.0±1.6 | 0.0±2.2 | 5.6±4.4 | 3.0±1.8 | 0.0±1.2 | 0.0±2.6 |
| Ⅱ-4 | 3.6±0.6 | 13.2±4.0 | 14.9±3.4 | 10.9±2.6 | 7.2±1.4 | 9.1±1.5 | 6.5±0.9 | 0.0±0.4 | 4.2±0.7 |
| Ⅱ-5 | 13.8±1.2 | 9.7±3.9 | 6.1±4.0 | 13.0±4.1 | 9.4±3.2 | 21.9±2.7 | 4.9±0.4 | 3.6±1.7 | 13.7±0.7 |
| Ⅱ-6 | 23.1±1.5 | 20.1±3.8 | 6.4±1.6 | 27.9±1.7 | 9.5±2.5 | 6.4±0.8 | 36.3±4.3 | 8.4±1.2 | 44.9±3.5 |
| Ⅱ-7 | 12.4±1.7 | 13.6±0.2 | 18.9±4.3 | 11.7±4.6 | 10.9±3.2 | 0.0±2.0 | 3.1±0.5 | 1.1±1.4 | 7.0±0.3 |
| Ⅱ-8 | 9.4±0.8 | 13.7±3.7 | 10.4±1.6 | 20.1±2.0 | 0.0±0.3 | 0.0±3.7 | 6.4±0.5 | 3.2±1.4 | 8.9±2.2 |
| Ⅱ-9 | 64.8±0.2 | 48.9±1.6 | 45.5±2.0 | 34.4±3.2 | 12.8±2.4 | 6.6±1.4 | 11.8±0.7 | 22.8±1.8 | 12.1±4.0 |
| Ⅱ-10 | 90.0±0.7 | 41.7±4.1 | 52.4±2.8 | 54.1±1.4 | 17.5±2.0 | 13.4±2.4 | 24.0±1.1 | 6.8±4.7 | 46.5±8.7 |
| Ⅱ-11 | 2.3±2.8 | 5.6±0.6 | 19.2±3.7 | 2.3±1.7 | 5.8±0.3 | 0.0±0.3 | 9.6±4.9 | 3.3±1.1 | 1.1±1.6 |
| Ⅱ-12 | 86.9±1.3 | 34.7±1.8 | 46.6±1.6 | 16.2±1.1 | 0.0±0.6 | 4.8±2.1 | 10.3±1.0 | 0.0±2.6 | 45.6±5.0 |
| 7Ⅰ-19 | 10.2±1..0 | 17.3±3.8 | 25.3±3.7 | 27.5±1.1 | 10.9±3.8 | 2.8±1.0 | 10.9±0.5 | 9.7±2.8 | 9.7±1.1 |
| 7Ⅰ-20 | 8.7±1.5 | 0.4±1.8 | 52.4±2.8 | 6.1±2.6 | 11.4±4.7 | 0.8±1.6 | 4.7±0.0 | 5.5±0.9 | 2.0±2.1 |
| 3 | 0.0±3.8 | 9.4±4.6 | 2.3±3.9 | 0.0±1.2 | 15.7±1.1 | 0.0±0.5 | 5.5±1.1 | 0.6±1.6 | 4.1±0.3 |
| Hymexazol | 100.0±0.0 | 50.4±1.9 | 25.8±2.0 | 1.5±1.2 | 37.5±0.4 | 41.4±0.7 | 43.0±1.3 | 51.6±0.3 | 36.4±2.0 |
| Compd. | Inhibition rate/% (50 μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PA | BC | RS | PC | CG | FG | MG | FO | FM | |
| Ⅰ-1 | 0.0±0.8 | 14.5±3.7 | 30.0±2.7 | 6.6±0.8 | 8.0±4.6 | 1.0±2.0 | 11.1±1.0 | 0.1±2.4 | 5.1±0.2 |
| Ⅰ-2 | 0.0±1.8 | 11.2±1.6 | 20.5±2.0 | 0.6±0.8 | 5.9±3.1 | 12.1±4.1 | 6.8±1.4 | 1.0±0.5 | 2.4±1.4 |
| Ⅰ-3 | 3.6±0.4 | 11.3±3.2 | 16.9±1.6 | 3.4±1.1 | 0.0±0.4 | 14.9±0.7 | 1.6±0.7 | 0.3±0.9 | 0.2±3.9 |
| Ⅰ-4 | 0.0±0.9 | 13.3±1.1 | 11.6±3.7 | 0.0±1.4 | 11.8±0.8 | 13.6±8.8 | 4.1±0.3 | 2.3±4.2 | 1.3±3.3 |
| Ⅰ-5 | 3.2±1.8 | 6.5±1.3 | 7.6±4.3 | 8.3±0.2 | 5.8±1.1 | 0.7±3.6 | 5.6±0.4 | 3.7±3.5 | 4.2±1.4 |
| Ⅰ-6 | 4.8±0.8 | 10.7±2.8 | 14.0±1.6 | 15.5±1.1 | 13.3±0.6 | 0.0±1.2 | 5.4±1.2 | 0.1±0.3 | 2.4±1.6 |
| Ⅰ-7 | 5.4±0.4 | 13.8±1.4 | 13.3±2.4 | 20.8±0.7 | 10.9±0.3 | 0.9±0.4 | 4.1±0.7 | 0.2±0.7 | 4.2±1.7 |
| Ⅰ-8 | 12.3±2.7 | 14.8±1.0 | 10.4±4.6 | 11.5±1.6 | 6.1±3.1 | 0.0±0.2 | 6.2±1.2 | 5.4±1.4 | 6.8±4.9 |
| Ⅰ-9 | 68.2±1.0 | 35.5±0.9 | 34.1±4.7 | 50.8±2.1 | 26.4±0.9 | 12.1±1.2 | 7.0±2.2 | 21.0±1.4 | 13.5±1.5 |
| Ⅰ-10 | 89.4±0.1 | 25.2±3.0 | 33.1±2.2 | 48.7±2.4 | 18.2±3.8 | 9.2±1.3 | 20.2±0.5 | 4.9±2.7 | 54.9±1.9 |
| Ⅰ-11 | 0.0±3.5 | 8.4±4.7 | 15.9±4.2 | 1.7±1.4 | 7.1±2.3 | 0.0±2.5 | 5.4±0.7 | 4.6±1.4 | 7.437±23 |
| Ⅰ-12 | 69.7±0.9 | 20.4±1.3 | 31.8±3.5 | 10.5±3.4 | 0.0±1.4 | 9.2±3.9 | 6.1±3.4 | 13.3±1.9 | 42.1±1.0 |
| Ⅰ-13 | 75.8±1.1 | 26.5±1.5 | 23.0±1.6 | 25.3±0.9 | 11.5±2.2 | 19.0±2.5 | 7.5±2.8 | 15.3±1.8 | 47.9±3.3 |
| Ⅰ-14 | 45.7±3.9 | 16.7±2.9 | 19.3±1.7 | 14.8±2.2 | 0.0±1.4 | 17.7±1.5 | 5.8±0.7 | 8.7±2.7 | 32.2±2.5 |
| Ⅰ-15 | 87.7±0.5 | 61.2±3.4 | 59.3±2.3 | 44.0±1.2 | 21.2±4.2 | 17.2±0.5 | 10.7±3.1 | 22.7±2.3 | 59.0±0.7 |
| Ⅰ-16 | 0.0±0.8 | 15.5±3.5 | 21.0±1.9 | 6.3±0.7 | 3.7±0.9 | 8.8±2.1 | 10.4±1.2 | 5.4±0.4 | 37.6±1.6 |
| Ⅰ-17 | 0.0±2.7 | 11.6±0.9 | 21.2±1.1 | 5.3±0.6 | 10.1±2.5 | 1.5±1.2 | 2.5±1.0 | 10.3±2.6 | 6.3±0.3 |
| Ⅰ-18 | 0.0±2.2 | 10.8±2.6 | 23.9±3.7 | 0.0±0.7 | 4.7±4.6 | 9.5±2.1 | 4.9±1.8 | 1.5±1.7 | 36.1±4.5 |
| Ⅱ-1 | 0.0±1.5 | 10.4±1.7 | 5.1±1.9 | 2.4±3.2 | 15.5±0.8 | 0.4±0.7 | 5.8±0.4 | 10.6±1.4 | 3.8±1.8 |
| Ⅱ-2 | 2.0±2.0 | 6.1±4.7 | 12.4±3.4 | 0.9±0.7 | 16.8±0.4 | 4.1±2.4 | 3.3±0.7 | 4.5±3.8 | 1.5±2.2 |
| Ⅱ-3 | 0.0±2.0 | 8.6±2.9 | 21.2±4.8 | 0.0±1.6 | 0.0±2.2 | 5.6±4.4 | 3.0±1.8 | 0.0±1.2 | 0.0±2.6 |
| Ⅱ-4 | 3.6±0.6 | 13.2±4.0 | 14.9±3.4 | 10.9±2.6 | 7.2±1.4 | 9.1±1.5 | 6.5±0.9 | 0.0±0.4 | 4.2±0.7 |
| Ⅱ-5 | 13.8±1.2 | 9.7±3.9 | 6.1±4.0 | 13.0±4.1 | 9.4±3.2 | 21.9±2.7 | 4.9±0.4 | 3.6±1.7 | 13.7±0.7 |
| Ⅱ-6 | 23.1±1.5 | 20.1±3.8 | 6.4±1.6 | 27.9±1.7 | 9.5±2.5 | 6.4±0.8 | 36.3±4.3 | 8.4±1.2 | 44.9±3.5 |
| Ⅱ-7 | 12.4±1.7 | 13.6±0.2 | 18.9±4.3 | 11.7±4.6 | 10.9±3.2 | 0.0±2.0 | 3.1±0.5 | 1.1±1.4 | 7.0±0.3 |
| Ⅱ-8 | 9.4±0.8 | 13.7±3.7 | 10.4±1.6 | 20.1±2.0 | 0.0±0.3 | 0.0±3.7 | 6.4±0.5 | 3.2±1.4 | 8.9±2.2 |
| Ⅱ-9 | 64.8±0.2 | 48.9±1.6 | 45.5±2.0 | 34.4±3.2 | 12.8±2.4 | 6.6±1.4 | 11.8±0.7 | 22.8±1.8 | 12.1±4.0 |
| Ⅱ-10 | 90.0±0.7 | 41.7±4.1 | 52.4±2.8 | 54.1±1.4 | 17.5±2.0 | 13.4±2.4 | 24.0±1.1 | 6.8±4.7 | 46.5±8.7 |
| Ⅱ-11 | 2.3±2.8 | 5.6±0.6 | 19.2±3.7 | 2.3±1.7 | 5.8±0.3 | 0.0±0.3 | 9.6±4.9 | 3.3±1.1 | 1.1±1.6 |
| Ⅱ-12 | 86.9±1.3 | 34.7±1.8 | 46.6±1.6 | 16.2±1.1 | 0.0±0.6 | 4.8±2.1 | 10.3±1.0 | 0.0±2.6 | 45.6±5.0 |
| 7Ⅰ-19 | 10.2±1..0 | 17.3±3.8 | 25.3±3.7 | 27.5±1.1 | 10.9±3.8 | 2.8±1.0 | 10.9±0.5 | 9.7±2.8 | 9.7±1.1 |
| 7Ⅰ-20 | 8.7±1.5 | 0.4±1.8 | 52.4±2.8 | 6.1±2.6 | 11.4±4.7 | 0.8±1.6 | 4.7±0.0 | 5.5±0.9 | 2.0±2.1 |
| 3 | 0.0±3.8 | 9.4±4.6 | 2.3±3.9 | 0.0±1.2 | 15.7±1.1 | 0.0±0.5 | 5.5±1.1 | 0.6±1.6 | 4.1±0.3 |
| Hymexazol | 100.0±0.0 | 50.4±1.9 | 25.8±2.0 | 1.5±1.2 | 37.5±0.4 | 41.4±0.7 | 43.0±1.3 | 51.6±0.3 | 36.4±2.0 |
| Compd. | Log P | Comd. | Log P | Compd. | Log P |
|---|---|---|---|---|---|
| Ⅰ-1 | 2.56 | Ⅰ-9 | 5.64 | Ⅰ-13 | 5.06 |
| Ⅰ-3 | 3.81 | Ⅰ-10 | 6.55 | Ⅰ-14 | 4.78 |
| Ⅰ-6 | 4.72 | Ⅰ-12 | 5.26 | Ⅰ-15 | 6.52 |
| Compd. | Log P | Comd. | Log P | Compd. | Log P |
|---|---|---|---|---|---|
| Ⅰ-1 | 2.56 | Ⅰ-9 | 5.64 | Ⅰ-13 | 5.06 |
| Ⅰ-3 | 3.81 | Ⅰ-10 | 6.55 | Ⅰ-14 | 4.78 |
| Ⅰ-6 | 4.72 | Ⅰ-12 | 5.26 | Ⅰ-15 | 6.52 |
| [1] |
Elbert, A.; Becker, B.; Hartwig, J.; Erdelen, C. Pflanzenschutz- Nachr. Bayer 1991, 44, 113.
|
| [2] |
Casida, J. E.; Quistad, G. B. Annu. Rev. Entomol. 1998, 43, 1.
pmid: 9444749 |
| [3] |
Jeschke, P.; Nauen, R. Pest Manage. Sci. 2008, 64, 1084.
doi: 10.1002/ps.v64:11 |
| [4] |
Tomizawa, M.; Casida, J. E. Acc. Chem. Res. 2009, 42, 260.
doi: 10.1021/ar800131p |
| [5] |
Casida, J. E. J. Agric. Food Chem. 2011, 59, 2923.
doi: 10.1021/jf102438c |
| [6] |
Kagabu, S. J. Agric. Food Chem. 2011, 59, 2887.
doi: 10.1021/jf101824y |
| [7] |
Tomizawa, M.; Casida, J. E. J. Agric. Food Chem. 2011, 59, 2883.
doi: 10.1021/jf103856c |
| [8] |
Jeschke, P.; Nauen, R.; Beck, M. E. Angew. Chem., Int. Ed. 2013, 52, 9464.
doi: 10.1002/anie.v52.36 |
| [9] |
Leite, S. A.; Guedes, R. N. C.; da Costa, D. R.; Colmenarez, Y. C.; Matsumoto, S. N.; dos Santos, M. P.; Coelho, B. S.; Moreira, A. A.; Castellani, M. A. Pest Manage. Sci. 2022, 78, 2581.
doi: 10.1002/ps.v78.6 |
| [10] |
Alsafran, M.; Rizwan, M.; Usman, K.; Saleem, M. H.; Jabri, H. A. J. Environ. Chem. Eng. 2022, 10, 108485.
doi: 10.1016/j.jece.2022.108485 |
| [11] |
Britt, E. E. Chem. Eng. News 2023, 101, 14.
|
| [12] |
Lourencetti, A. P. S.; Azevedo, P.; Miotelo, L.; Malaspina, O.; Nocelli, R. C. F. Environ. Pollut. 2023, 318, 120842.
doi: 10.1016/j.envpol.2022.120842 |
| [13] |
European Food Safety, A. EFSA J. 2018, 16, e05178.
|
| [14] |
Demortain, D. Curr. Opin. Insect. Sci. 2021, 46, 78-82.
doi: 10.1016/j.cois.2021.02.017 pmid: 33737144 |
| [15] |
Onozaki, Y.; Horikoshi, R.; Ohno, I.; Kitsuda, S.; Durkin, K. A.; Suzuki, T.; Asahara, C.; Hiroki, N.; Komabashiri, R.; Shimizu, R.; Furutani, S.; Ihara, M.; Matsuda, K.; Mitomi, M.; Kagabu, S.; Uomoto, K.; Tomizawa, M. J. Agric. Food Chem. 2017, 65, 7865.
doi: 10.1021/acs.jafc.7b02924 |
| [16] |
Xu, Y.; Yang, D. Y.; Zou, X. G.; Rui, C. H.; Zhou, Z. Y.; Ma, Y. Q.; Qin, Z. H. Pest Manage. Sci. 2017, 73, 1927.
doi: 10.1002/ps.2017.73.issue-9 |
| [17] |
Yang, S.; Lai, Q.; Lai, F.; Jiang, X.; Zhao, C.; Xu, H. Pest Manage. Sci. 2021, 77, 1013.
doi: 10.1002/ps.v77.2 |
| [18] |
Nauen, R.; Jeschke, P.; Velten, R.; Beck, M. E.; Ebbinghaus- Kintscher, U.; Thielert, W.; Wolfel, K.; Haas, M.; Kunz, K.; Raupach, G. Pest Manage. Sci. 2015, 71, 850.
doi: 10.1002/ps.2015.71.issue-6 |
| [19] |
Wernecke, A.; Eckert, J. H.; Forster, R.; Kurlemann, N.; Odemer, R. J. Plant Dis. Protect. 2021, 129, 93.
|
| [20] |
Tamburini, G.; Wintermantel, D.; Allan, M. J.; Dean, R. R.; Knauer, A.; Albrecht, M.; Klein, A. M. Sci. Total Environ. 2021, 778, 146084.
doi: 10.1016/j.scitotenv.2021.146084 |
| [21] |
Jeschke, P.; Nauen, R.; Gutbrod, O.; Beck, M. E.; Matthiesen, S.; Haas, M.; Velten, R. Pest. Biochem. Physiol. 2015, 121, 31.
doi: 10.1016/j.pestbp.2014.10.011 |
| [22] |
Zhu, C.; Li, G.; Xiao, K.; Shao, X.; Cheng, J.; Li, Z. Chin. Chem. Lett. 2019, 30, 255.
doi: 10.1016/j.cclet.2018.05.013 |
| [23] |
Markussen, M. D. K.; Kristensen, M. Pest. Biochem. Physiol. 2010, 98, 50.
doi: 10.1016/j.pestbp.2010.04.012 |
| [24] |
Kim, J.; Chon, K.; Kim, B.-S.; Oh, J.-A.; Yoon, C.-Y.; Park, H.-H. Pest Manage. Sci. 2022, 78, 5402.
doi: 10.1002/ps.v78.12 |
| [25] |
Zhang, D.; Zhang, J.; Liu, T.; Wu, S.; Wu, Z.; Wu, S.; Song, R.; Song, B. J. Agric. Food Chem. 2022, 70, 8598.
doi: 10.1021/acs.jafc.2c01899 |
| [26] |
Sur, R. B. Insectol. 2003, 56, 35.
|
| [27] |
Nauen, R.; Tietjen, K.; Wagner, K.; Elbert, A. Pestic. Sci. 1998, 52, 53.
doi: 10.1002/(SICI)1096-9063(199801)52:1【-逻*辑*与-】amp;lt;【-逻*辑*与-】amp;gt;1.0.CO;2-J |
| [28] |
Nauen, R.; Reckmann, U.; Armborst, S.; Stupp, H.-P.; Elbert, A. Pestic. Sci. 1999, 55, 265.
doi: 10.1002/(SICI)1096-9063(199903)55:3【-逻*辑*与-】amp;lt;【-逻*辑*与-】amp;gt;1.0.CO;2-5 |
| [29] |
Liu, J.; Zhang, Y.; Dong, F.; Wu, X.; Pan, X.; Xu, J.; Zheng, Y. J. Sep. Sci. 2022, 45, 3567.
doi: 10.1002/jssc.v45.18 |
| [30] |
Suchail, S.; Guez, D.; Belzunces, L. P. Environ. Toxicol. Chem. 2001, 20, 2482.
doi: 10.1897/1551-5028(2001)020【-逻*辑*与-】lt;2482:dbaact【-逻*辑*与-】gt;2.0.co;2 pmid: 11699773 |
| [31] |
Jayashree, B. S.; Nikhil, P. S.; Paul, S. Med. Chem. 2022, 18, 915-925.
doi: 10.2174/1573406418666220127124228 |
| [32] |
Kang, O.-Y.; Kim, E.; Lee, W. H.; Ryu, D. H.; Lim, H. J.; Park, S. J. RSC Adv. 2023, 13, 2004.
doi: 10.1039/D2RA06988A |
| [33] |
Wu, H.; Maimaitijiang, A.; Tang, D.; Xie, B.; Niu, C.; Aisa, H. A. Heterocycles 2023, 106, 94.
doi: 10.3987/COM-22-14764 |
| [34] |
Guzel, E.; Acar Cevik, U.; Evren, A. E.; Bostanc, H. E.; Gul, U. D.; Kays, U.; Ozkay, Y.; Kaplanckl, Z. A. ACS Omega 2023, 8, 4369.
doi: 10.1021/acsomega.2c07755 |
| [35] |
Li, J.; Zhang, J. Curr. Top. Med. Chem. 2022, 22, 41.
doi: 10.2174/1568026621666211111160332 |
| [36] |
Gupta, O.; Pradhan, T.; Chawla, G. J. Mol. Struct. 2023, 1274, 134487.
doi: 10.1016/j.molstruc.2022.134487 |
| [37] |
Xie, R.; Mei, X.; Ning, J. Chem. Pharm. Bull. 2019, 67, 345.
doi: 10.1248/cpb.c18-00704 |
| [38] |
Li, B.; Yu, Y.; Duan, W.; Lin, G.; Wen, R.; Zhang, Z. Chem. Biodivers. 2022, 19, e202200726.
doi: 10.1002/cbdv.v19.11 |
| [39] |
Zhao, F.; Singh, T.; Xiao, Y.; Su, W.; Yang, D.; Jia, C.; Li, J.-Q.; Qin, Z. Synthesis 2021, 53, 1901.
doi: 10.1055/a-1477-4630 |
| [40] |
Ouyang, Y.; Huang, J.-j.; Wang, Y.-L.; Zhong, H.; Song, B.-A.; Hao, G.-f. J. Agric. Food Chem. 2021, 69, 10761.
doi: 10.1021/acs.jafc.1c01460 |
| [41] |
Chen, D.; Hao, G.; Song, B. J. Agric. Food Chem. 2022, 70, 10090.
doi: 10.1021/acs.jafc.2c02757 |
| [42] |
Banerjee, P.; Eckert, A. O.; Schrey, A. K.; Preissner, R. Nucleic Acids Res. 2018, 46, W257.
doi: 10.1093/nar/gky318 |
| [43] |
Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P. W.; Tang, Y. J. Chem. Inf. Model. 2012, 52, 3099.
doi: 10.1021/ci300367a |
| [44] |
Williams, A. J.; Grulke, C. M.; Edwards, J.; McEachran, A. D.; Mansouri, K.; Baker, N. C.; Patlewicz, G.; Shah, I.; Wambaugh, J. F.; Judson, R. S.; Richard, A. M. J. Cheminf. 2017, 9, 61/61-61/27.
|
| [45] |
Perez Santin, E.; Rodriguez Solana, R.; Gonzalez Garcia, M.; Garcia Suarez, M. D. M.; Blanco Diaz, G. D.; Cima Cabal, M. D.; Moreno Rojas, J. M.; Lopez Sanchez, J. I. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2021, 11, e1516.
|
| [46] |
Han, Q.; Wu, N.; Liu, Y.-Y.; Zhang, J.-Y.; Zhang, R.-L.; Li, H.-L.; Jiang, Z.-Y.; Huang, J.-X.; Duan, H.-X.; Yang, Q. J. Agric. Food Chem. 2022, 70, 7387.
doi: 10.1021/acs.jafc.2c02091 |
| [47] |
Zhao, P.-L.; Wang, F.; Zhang, M.-Z.; Liu, Z.-M.; Huang, W.; Yang, G.-F. J. Agric. Food Chem. 2008, 56, 10767.
doi: 10.1021/jf802343p |
| [48] |
Su, W.; Zhou, Y.; Ma, Y.; Wang, L.; Zhang, Z.; Rui, C.; Duan, H.; Qin, Z. J. Agric. Food Chem. 2012, 60, 5028.
doi: 10.1021/jf300616x |
| [49] |
Wang, J.; Liu, X.; Zhang, X.; Du, S.; Han, X.; Li, J. Q.; Xiao, Y.; Xu, Z.; Wu, Q.; Xu, L.; Qin, Z. J. Agric. Food Chem. 2022, 70, 111.
doi: 10.1021/acs.jafc.1c05784 |
| [1] | Simin Wu, Jiaxin Tang, Yujia Zhou, Xuetao Xu, Haoxing Zhang, Shaohua Wang. α-Glucosidase Inhibition Research of Derivatives Based on 2β-Acetoxyferruginol Scaffold Excluding Acetic Acid Group [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 613-621. |
| [2] | Penghui Li, Qingyang Xie, Fuxian Wan, Yuanhong Zhang, Lin Jiang. Synthesis and Fungicidal Activity of Novel Substituted Pyrimidine-5-carboxamides Bearing Cyclopropyl Moiety [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 650-656. |
| [3] | Feng Wang, Yu Chen, Hongyan Pei, Jing Zhang, Lixin Zhang. Design, Synthesis and Antifungal Activities of Novel 1,2,4-Oxadiazole Derivatives Containing Piperidine [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2826-2836. |
| [4] | Zichan Zhang, Yang Sun, Sheng Hua, Baolin Xu, Min Zhang, Qin Zhao, Dandan Zheng, Yang Wang, Jianfeng Ju, Yujun Shi, Hong Dai. Synthesis and Insecticidal Activity of Novel Pyrazole Amides Containing an Isoxazole Moiety [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1435-1443. |
| [5] | Huan Xu, Hongfei Wu, Xiaoming Zhang, Xingxing Lu, Tengda Sun, Yue Qi, Yufan Lin, Xinling Yang, Li Zhang, Yun Ling. Design, Synthesis and Bioactivity of Sulfonyl Hydrazides and Hydrazides Containing Fragment 1,2,3,4-Tetrahydroisoquinoline [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 725-733. |
| [6] | Lei Wang, Shujing Yu, Na Yang, Baolei Wang. Studies on the Synthesis and Biological Activities of Novel Dihydroquinazolinone-Containing Caffeine Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 299-307. |
| [7] | Changxing Sun, Fuhao Zhang, Huan Zhang, Penghui Li, Lin Jiang. Design, Synthesis, Fungicidal Activity and Molecular Docking Study of Novel 2-(1-Methyl-1H-pyrazol-4-yl)pyrimidine-4-carboxamides [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 229-235. |
| [8] | Wenjuan Li, Rui Zhang, Zhihua Cai, Xiaoqiang Han, Lin He, Bin Dai. Constrution and Insecticidal Activities of Trifluoromethylated Benzocyclicsulfoximine Derivatives by [3+2] Cycloaddition Reaction of Beznyne [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2832-2839. |
| [9] | Qian Zhang, Yihao Li, Leichuan Xu, Haoyun Ma, Xiangdong Li, Ming'an Wang. Synthesis and Fungicidal Activity of Novel Butenolide Compounds Containing Oxime Ether Moiety [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2438-2448. |
| [10] | Changkai Wang, Tengda Sun, Xuebo Zhang, Xinling Yang, Xingxing Lu, Huan Xu, Fasheng Shi, Li Zhang, Yun Ling. Design, Synthesis and Bioactivity of Novel Fluoropyrazole Hydrazides [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1527-1536. |
| [11] | Xiu Wang, Wengui Duan, Guishan Lin, Baoyu Li, Wenjing Zhang, Fuhou Lei. Synthesis, Antifungal Activity, Three-Dimensional Quantitative Structure-Activity Relationship and Molecular Docking Study of 4-Acyl-3-amino-1,2,4-triazole-thioether Derivatives Containing Natural Pinene Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 871-883. |
| [12] | Yuanfang Kong, Bin Yang, Yan Zhuang, Jingyu Zhang, Demei Sun, Chunhong Dong. Research Progress on the Synthesis and Structure-Activity Relationship of Five Hypoglycemic Active Heterocycles Based on Dipeptidyl Peptidase 4 (DPP-4) Target Design [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 770-784. |
| [13] | Yan Zeng, Lifei Nie, Chao Niu, Aytilla Mamatjan, Khurshed Bozorov, Jiangyu Zhao, Haji Akber Aisa. Synthesis and Biological Activities of Dihydrooxazolo[5,4-d]-pyrrolo[1,2-a]pyrimidinones [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 543-556. |
| [14] | Shu Chen, Yingying Shao, Xinhao Fu, Qingwu Chen, Xiaohua Du, Chengxia Tan. Design, Synthesis and Insecticidal Activities of Pyridyl Thiazole Diamide Compounds [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3870-3879. |
| [15] | Yucheng Cui, Meihua Chen, Guishan Lin, Wengui Duan, Qingmin Li, Renxuan Zou, Bo Cen. Synthesis, Antifungal Activity and Molecular Docking Study of 1,3,4-Thiadiazole-Urea Compounds Containing gem-Dimethylcyclopropane Ring Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3784-3797. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||