Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (9): 3287-3296.DOI: 10.6023/cjoc202302025 Previous Articles Next Articles
曾崇洋a, 胡平a, 汪必琴a, 方文彦b, 赵可清a,*( )
)
收稿日期:2023-02-23
修回日期:2023-04-10
发布日期:2023-05-05
基金资助:
Chongyang Zenga, Ping Hua, Biqin Wanga, Wenyan Fangb, Keqing Zhaoa( )
)
Received:2023-02-23
Revised:2023-04-10
Published:2023-05-05
Contact:
E-mail: Supported by:Share
Chongyang Zeng, Ping Hu, Biqin Wang, Wenyan Fang, Keqing Zhao. Cyanostilbene Bridged Triphenylene Dyad Stimuli-Responsive Discotic Liquid Crystal: Synthesis, Properties and Applications[J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3287-3296.
| Compd. | Solvent | λa/nm | εb/(×104 L•cm–1•mol–1) | λec/nm | QYd/% | Δνe/cm–1 | Δfh |
|---|---|---|---|---|---|---|---|
| PBTPA | CHX | 376 | 19.62 | 460 | 73.85 | 4857 | 0.0095 |
| CCl4 | 374 | 21.27 | 468 | 45.17 | 5730 | 0.0029 | |
| TOL | 376 | 9.41 | 474 | 33.25 | 5499 | 0.0171 | |
| DOX | 372 | 24.98 | 487 | 29.12 | 6348 | 0.0246 | |
| EA | 368 | 21.31 | 523 | 12.74 | 7907 | 0.1996 | |
| EtOH | 378 | 22.16 | 550 | 11.18 | 8273 | 0.2901 | |
| DMF | 384 | 24.12 | 570 | — | 8497 | 0.2751 |
| Compd. | Solvent | λa/nm | εb/(×104 L•cm–1•mol–1) | λec/nm | QYd/% | Δνe/cm–1 | Δfh |
|---|---|---|---|---|---|---|---|
| PBTPA | CHX | 376 | 19.62 | 460 | 73.85 | 4857 | 0.0095 |
| CCl4 | 374 | 21.27 | 468 | 45.17 | 5730 | 0.0029 | |
| TOL | 376 | 9.41 | 474 | 33.25 | 5499 | 0.0171 | |
| DOX | 372 | 24.98 | 487 | 29.12 | 6348 | 0.0246 | |
| EA | 368 | 21.31 | 523 | 12.74 | 7907 | 0.1996 | |
| EtOH | 378 | 22.16 | 550 | 11.18 | 8273 | 0.2901 | |
| DMF | 384 | 24.12 | 570 | — | 8497 | 0.2751 |
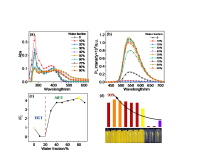



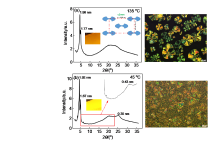
| Compd. | Process | Phase transitionb/℃ [∆H/(kJ•mol–1)] |
|---|---|---|
| PBTPA | 2nd heating | Cr 128 (11.56) Colrec 158 (4.55) I |
| 1st cooling | I 144 (11.78) Colrec 119 (15.91) Cr |
| Compd. | Process | Phase transitionb/℃ [∆H/(kJ•mol–1)] |
|---|---|---|
| PBTPA | 2nd heating | Cr 128 (11.56) Colrec 158 (4.55) I |
| 1st cooling | I 144 (11.78) Colrec 119 (15.91) Cr |


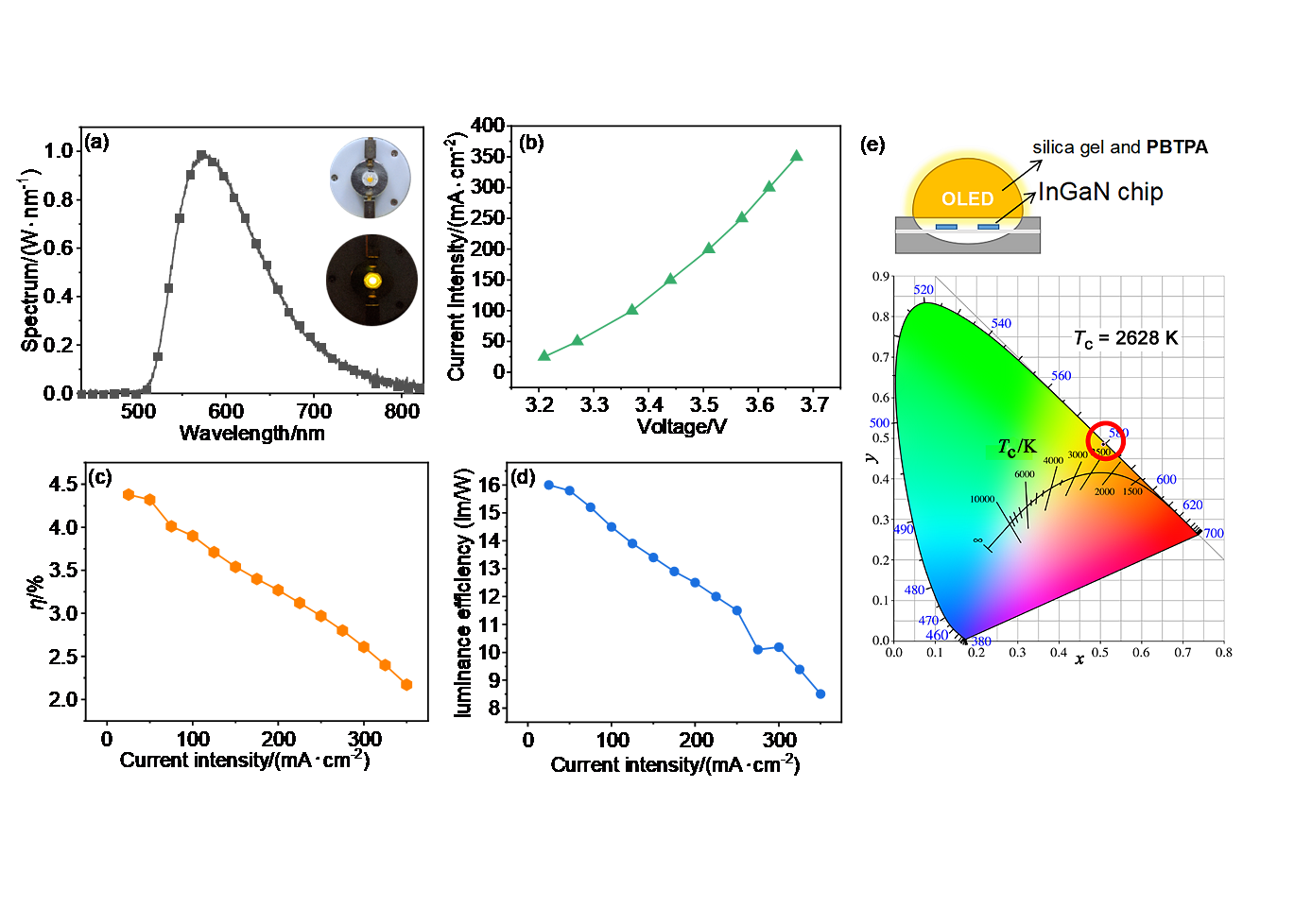
| [1] |
Hari K. B.; Li Q. Chem. Rev. 2022, 122, 4887.
doi: 10.1021/acs.chemrev.1c00761 |
| [2] |
Sandeep K. Liq. Cryst. 2004, 31, 1037.
doi: 10.1080/02678290410001724746 |
| [3] |
Yin D.; Shang H.-Y.; Yu W.-H.; Xiang S.-K.; Hu P.; Zhao K.-Q.; Feng C; Wang B.-Q. Acta Chim. Sinica 2022, 80, 1376. (in Chinese)
doi: 10.6023/A22070288 |
|
(殷东, 商宏怡, 余文浩, 向仕凯, 胡平, 赵可清, 冯春, 汪必琴, 化学学报, 2022, 80, 1376.)
doi: 10.6023/A22070288 |
|
| [4] |
Li Z.-M.; Shuai B.; Mei C.; Fang P.; Mei T.-S. Chin. J. Chem. 2022, 40, 2335.
doi: 10.1002/cjoc.v40.19 |
| [5] |
Yang F.-F.; Xie J.-W.; Guo H.-Y.; Xu B.-T.; Li C.-C. Liq. Cryst. 2012, 39, 1368.
doi: 10.1080/02678292.2012.717112 |
| [6] |
Yu W.-H.; Chen C.; Hu P.; Wang B.-Q.; Carl R.; Zhao K.-Q. RSC Adv. 2013, 3, 14099.
doi: 10.1039/c3ra41874j |
| [7] |
Lin H.; Zhao K.-X.; Jing M.; Long X.-H.; Zhao K.-Q.; Hu P.; Wang B.-Q.; Lei P.; Zeng Q.-D.; Bertrand D. J. Mater. Chem. C 2022, 10, 14453.
doi: 10.1039/D2TC02441A |
| [8] |
Wang L.; Zhang R.; Huang Z.; Guo S.-M.; Yang J.-X.; Kong L. Dyes Pigm. 2022, 197, 109909.
doi: 10.1016/j.dyepig.2021.109909 |
| [9] |
Lin L.-B.; Guo H.-Y.; Fang X.-T.; Yang F.-F. RSC Adv. 2017, 7, 20172.
doi: 10.1039/C7RA02338C |
| [10] |
Hu R.-R.; Nelson L. C. L.; Tang B.-Z. Chem. Soc. Rev. 2014, 4, 4494.
|
| [11] |
Mei J.; Nelson L. C. L.; Tsz K. K.; Wing Y. L.; Tang B.-Z. Chem. Rev. 2015, 115, 11718.
doi: 10.1021/acs.chemrev.5b00263 pmid: 26492387 |
| [12] |
Yan Q.; Wang S. Mater. Chem. Front. 2020, 4, 3153.
doi: 10.1039/D0QM00522C |
| [13] |
(a) Zhao D.-Y. ACS Symp. Ser. 2016, 2, 152.
|
|
(b) Kang J.-X.; Yu J.; Li A.-R.; Zhao D.-Y; Liu B.; Guo L.; Tang B.-Z. iScience 2019, 15, 126.
|
|
| [14] |
Wang F.; Li X.; Wang S.; Li C.-P.; Dong D.; Ma X.; Kim S. H.; Cao D.-R. Chin. Chem. Lett. 2016, 27, 1592.
doi: 10.1016/j.cclet.2016.04.020 |
| [15] |
Fang W.-Y.; Zhang G.-B.; Chen J.; Yang L.-M.; Kong L.; Yang J.-X. Sens. Actuators, B 2016, 229, 338.
doi: 10.1016/j.snb.2016.01.130 |
| [16] |
Yu X.-W.; Zhang H.-Y.; Yu J.-H. Aggregate 2021, 2, 20.
doi: 10.1002/agt2.v2.1 |
| [17] |
Fan T.-W.; Chen X.-H.; Liu D.-H; Su S.-J.; Guo H.-Y.; Yang F.-F. J. Mater. Chem. C 2022, 10, 5598.
doi: 10.1039/D2TC00461E |
| [18] |
Guo H.-Y.; Yu Q.; Xiong Y.-Z.; Yang F.-F. J. Mol. Liq. 2021, 335, 116179.
doi: 10.1016/j.molliq.2021.116179 |
| [19] |
Guo H.-Y.; Lin L.-B.; Qiu J.-B.; Yang F.-F. RSC Adv. 2017, 7, 53316.
doi: 10.1039/C7RA10218F |
| [20] |
Jong W. C.; Seong-Jun Y.; Byeong-Kwan A.; Soo Y. P. J. Phys. Chem. C 2013, 117, 11285.
doi: 10.1021/jp401440s |
| [21] |
Lu H.-B.; Zhang S.-N.; Ding A.-X.; Yuan M.; Zhang G.-Y.; Xu W.; Zhang G.-B.; Wang X.-H.; Qiu L.-Z.; Yang J.-X. New J. Chem. 2014, 38, 3429.
doi: 10.1039/C4NJ00218K |
| [22] |
Bao R.; Pan M.; Qiu J.-J.; Liu C.-M. Chin. Chem. Lett. 2010, 21, 682.
doi: 10.1016/j.cclet.2009.12.020 |
| [23] |
Zeng C.-Y.; Cao Z.; He Y.-R.; Ye T.-T.; Gao Y.-S.; Li D.-H.; Liu Q.-M.; Zhou W.-W.; Fang W.-Y. Results Optics 2022, 8, 100264.
doi: 10.1016/j.rio.2022.100264 |
| [24] |
(a) Fang W.-Y.; Zhao W.; Pei P.; Liu R.; Zhang Y.-Y; Kong L.; Yang J.-X. J. Mater. Chem. C 2018, 6, 9269.
doi: 10.1039/C8TC02973C |
|
(b) Ma T.; Chen, S.-B.; Du, X.-Y.; Mo, M.-S.; Cheng, X.-H. Dyes Pigm. 2023, 213, 111176.
doi: 10.1016/j.dyepig.2023.111176 |
|
| [25] |
Zhang J.; He B.-Z.; Hu Y.-B.; Parvej A.; Zhang H.-K.; Jacky W. Y. L.; Tang B.-Z. Adv. Mater. 2021, 33, 2008071.
doi: 10.1002/adma.v33.32 |
| [26] |
Xia Z.-G.; Liu Q.-L. Prog. Mater. Sci. 2016, 84, 59.
doi: 10.1016/j.pmatsci.2016.09.007 |
| [27] |
Kazunori T.; Shintaro N.; Norimasa Y.; Takuma Y.; Chihaya A. J. Mater. Chem. 2012, 22, 20689.
doi: 10.1039/c2jm33669c |
| [28] |
Seul O. K.; Heung S. J.; Seok J. L.; Young K. K.; Seung S. Y. Bull. Korean Chem. Soc. 2013, 34, 2267.
doi: 10.5012/bkcs.2013.34.8.2267 |
| [29] |
Nuttapong C.; Phattananawee N.; Pongsakorn C.; Wijitra W.; Taweesak S.; Vinich P. J. Lumin. 2022, 248, 118926.
doi: 10.1016/j.jlumin.2022.118926 |
| [30] |
Wu X.; Liu Y.-Q.; Zhu D.-B. J. Mater. Chem. 2001, 11, 1327.
doi: 10.1039/b008521i |
| [31] |
Mi D.-D.; Hee U. K.; Seon Y. L.; Jonghee L.; Sung-Chul H.; Sungmoon P.; Do-Hoon H. Mol. Cryst. Liq. Cryst. 2010, 530, 220.
|
| [32] |
(a) Fang W.-Y.; Zhang Y.-Y.; Chen J.; Yang L.-M.; Kong L.; Yang J.-X. CrystEngComm 2017, 19, 1294.
doi: 10.1039/C6CE02376B |
|
(b) Fang W.-Y.; Cao Z.; Liu Q.-M.; Chu Y.-H.; Zhu H.-F.; Zhou W.-W.; Yang J.-X. Results Optics 2022, 7, 100228.
doi: 10.1016/j.rio.2022.100228 |
|
| [33] |
(a) Deng W.-J.; Liu S.; Lin H.; Zhao K.-X.; Bai X.-Y.; Zhao K.-Q.; Hu P.; Wang B.-Q; Hirosato M.; Bertrand D. New J. Chem. 2022, 46, 7936.
doi: 10.1039/D2NJ00655C |
|
(b) Bai Y.-F.; Chen L.-Q.; Hu P.; Luo K.-J.; Yu W.-H.; Ni H.-L.; Zhao K.-Q.; Wang B.-Q. Liq. Cryst. 2015, 42, 1591.
|
|
|
(c) An L.-L.; Jing M.; Xiao B.; Bai X.-Y.; Zeng Q.-D.; Zhao K.-Q. Chin. Phys. B 2016, 25, 096402.
doi: 10.1088/1674-1056/25/9/096402 |
|
|
(d) Zhu X.-M.; Bai X.-Y.; Wang H.-F.; Zhao K.-Q.; Wang B.-Q. Acta Chim. Sinica 2021, 79, 1486. (in Chinese)
doi: 10.6023/A21080397 |
|
|
(朱雪敏, 白小燕, 王海峰, 赵可清, 汪必琴, 化学学报, 2021, 79, 1486.)
doi: 10.6023/A21080397 |
|
| [34] |
Liu T.-H.; Yang L.-J.; Liu K.; Ran Q.; Wang W.-N; Liu Q.; Peng H.-N.; Ding N.; Fang Y. ACS Appl. Mater. Interfaces 2020, 12, 11084.
doi: 10.1021/acsami.0c00568 |
| [35] |
Nicolas G.; Alessandra C.; Mauro G.; Manuela C.; Francesco C.; Andrea F. J. Appl. Cryst. 2014, 47, 668.
doi: 10.1107/S1600576714003240 |
| [36] |
Feng C.-F.; Li J.-Y.; Han X.; He X.; Liu L.-Q.; Li X.-X.; Sun X.-Y.; Lu P.; Ma Y.-G. Faraday Discuss. 2017, 196, 163.
doi: 10.1039/C6FD00159A |
| [1] | Yang Zhao, Panpan Chen, Lizhi Han, Enju Wang. Aggregation-Induced Emission and Cell Imaging of Triphenylimidazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2454-2461. |
| [2] | Yuehua Zhang, Fei Nie, Lu Zhou, Xiaofeng Wang, Yuan Liu, Yanping Huo, Wencheng Chen, Zujin Zhao. Synthesis and Optoelectronic Studies of Thermally Activated Delayed Fluorescence Materials Based on Benzothiazolyl Ketones [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3876-3887. |
| [3] | Meng Liu, Yanru Huang, Xiaofei Sun, Lijun Tang. An “Aggregation-Induced Emission+Excited-State Intramolecular Proton Transfer” Mechanisms-Based Benzothiazole Derived Fluorescent Probe and Its ClO– Recognition [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 345-351. |
| [4] | Yangyang Li, Xiaofei Sun, Xiaoling Hu, Yuanyuan Ren, Keli Zhong, Xiaomei Yan, Lijun Tang. Synthesis of Triphenylamine Derivative and Its Recognition for Hg2+ with “OFF-ON” Fluorescence Response Based on Aggregation-Induced Emission (AIE) Mechanism [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 320-325. |
| [5] | Jidong Zhang, Wanlin Yan, Wenqiang Hu, Dian Guo, Dalong Zhang, Xiaoxin Quan, Xianpan Bu, Siyu Chen. Design and Synthesis of a Zn2+ Fluorescent Probe Based on Aggregation Induced Luminescence Properties [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 326-331. |
| [6] | Zhaohua Chen, Xiying Cao, Sihong Chen, Shiwei Yu, Yanlan Lin, Shuting Lin, Zhaoyang Wang. Design, Synthesis and Application of Trisubstituted Olefinic Aggregation-Induced Emission Molecules [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2355-2363. |
| [7] | Ze Guo, Di Wu, Lili Wang, Zheng Duan. BF3•Et2O Promoted Dienone-Phenol Type Rearrangement to Synthesize Phosphepine with Aggregation Induced Luminescence (AIE) Effect [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2481-2487. |
| [8] | Yuetian Guo, Yongxin Pan, Lijun Tang. Progresses in Reactive Fluorescent Probes with Fused Aggregation- Induced Emission (AIE) and Excited State Intramolecular Proton Transfer (ESIPT) Structures [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1640-1650. |
| [9] | Sihong Chen, Jiamin Xu, Yuemei Li, Baoru Peng, Lingyu Luo, Huiye Feng, Zhaohua Chen, Zhaoyang Wang. Research Progress of Aggregation-Caused Quenching (ACQ) to Aggregation-Induced Emission (AIE) Transformation Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1651-1666. |
| [10] | Jiang Qian, Wang Zhonglong, Li Mingxin, Yang Yiqin, Xu Xu, Xu Haijun, Wang Shifa. Nopinone-Based Difluoroboron β-Diketonate Complex: Aggregation-Induced Emission and Solvatochromism [J]. Chinese Journal of Organic Chemistry, 2020, 40(12): 4290-4297. |
| [11] | Lin Danyan, Song Senchuan, Chen Zhiyong, Guo Pengran, Chen Jianghan, Shi Huahong, Mai Yuliang, Song Huacan. Luminescence Properties of the Conjugated System Containing Benzoimidazole Structural Units and Its Organic Light-Emitting Diode (OLED) [J]. Chin. J. Org. Chem., 2018, 38(1): 103-111. |
| [12] | Liu Ruijiao, Zeng Jing. Study on a Novel Colorimetric and Off-On Fluorescent Chemosensor with Aggregation-Induced Emission Effect for Detection of Fe3+ in Aqueous Solution [J]. Chin. J. Org. Chem., 2017, 37(12): 3274-3281. |
| [13] | Kong Xiangfei, Liu Peng, Wang Guixia, Xia Liting, Dai Shengping, Su Jian, Liao Peihai, Liu Zheng, Mu Linping. Synthesis and Properties of Alkoxy-Bridged Triphenylene and Perylene Monoimide Diesters Dyads [J]. Chin. J. Org. Chem., 2016, 36(6): 1325-1334. |
| [14] | Wang Wenli, He Qing, Ding Ru, He Yun, Zhang Zunting. Synthesis of 1-Hydroxy-2-phenyltriphenylene Derivatives by Three-Step Condensation [J]. Chin. J. Org. Chem., 2014, 34(9): 1875-1880. |
| [15] | LI Juan-Juan, HE Zhi-Qun, HU Min, KONG Xiang-Fei, ZHANG Chun-Xiu. A Synthetic Route towards a Crown-Ether Linked Triphenylene Dimer [J]. Chin. J. Org. Chem., 2010, 30(04): 590-594. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||