Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (1): 320-325.DOI: 10.6023/cjoc202206043 Previous Articles Next Articles
NOTES
李阳阳a, 孙小飞a,*( ), 胡晓玲a, 任源远b, 钟克利a,*(
), 胡晓玲a, 任源远b, 钟克利a,*( ), 燕小梅c, 汤立军a
), 燕小梅c, 汤立军a
收稿日期:2022-06-22
修回日期:2022-08-01
发布日期:2022-09-01
通讯作者:
孙小飞, 钟克利
基金资助:
Yangyang Lia, Xiaofei Suna( ), Xiaoling Hua, Yuanyuan Renb, Keli Zhonga(
), Xiaoling Hua, Yuanyuan Renb, Keli Zhonga( ), Xiaomei Yanc, Lijun Tanga
), Xiaomei Yanc, Lijun Tanga
Received:2022-06-22
Revised:2022-08-01
Published:2022-09-01
Contact:
Xiaofei Sun, Keli Zhong
Supported by:Share
Yangyang Li, Xiaofei Sun, Xiaoling Hu, Yuanyuan Ren, Keli Zhong, Xiaomei Yan, Lijun Tang. Synthesis of Triphenylamine Derivative and Its Recognition for Hg2+ with “OFF-ON” Fluorescence Response Based on Aggregation-Induced Emission (AIE) Mechanism[J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 320-325.

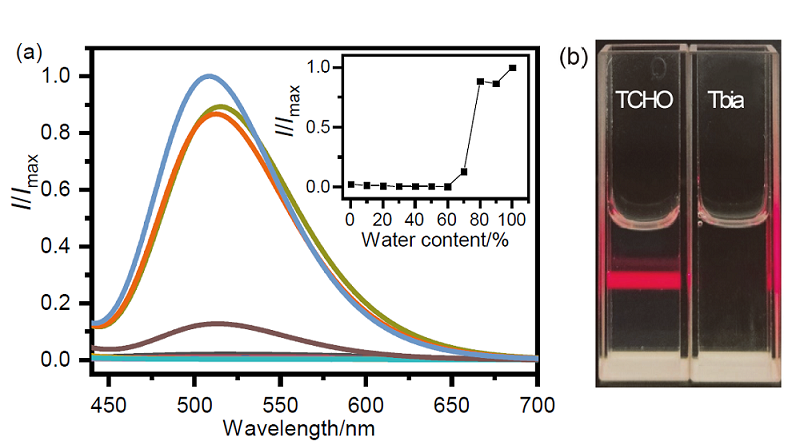

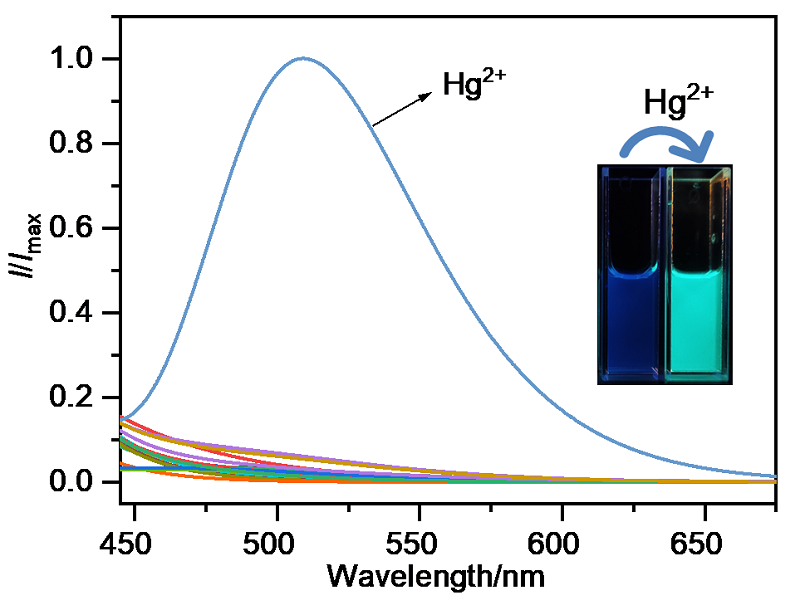


| Sample | Added/ (μmol•L–1) | Found/ (μmol•L–1) | Recovery rate/% | CV (n=3) |
|---|---|---|---|---|
| Tap water | 4 | 4.11 | 102.7 | 0.08 |
| 5 | 4.94 | 98.9 | 0.05 | |
| 6 | 5.97 | 99.5 | 0.06 | |
| Lake water | 4 | 3.98 | 99.5 | 0.02 |
| 5 | 5.04 | 100.7 | 0.02 | |
| 6 | 5.95 | 99.2 | 0.01 | |
| River water | 4 | 4.01 | 100.2 | 0.05 |
| 5 | 4.90 | 98.0 | 0.09 | |
| 6 | 6.07 | 101.2 | 0.04 |
| Sample | Added/ (μmol•L–1) | Found/ (μmol•L–1) | Recovery rate/% | CV (n=3) |
|---|---|---|---|---|
| Tap water | 4 | 4.11 | 102.7 | 0.08 |
| 5 | 4.94 | 98.9 | 0.05 | |
| 6 | 5.97 | 99.5 | 0.06 | |
| Lake water | 4 | 3.98 | 99.5 | 0.02 |
| 5 | 5.04 | 100.7 | 0.02 | |
| 6 | 5.95 | 99.2 | 0.01 | |
| River water | 4 | 4.01 | 100.2 | 0.05 |
| 5 | 4.90 | 98.0 | 0.09 | |
| 6 | 6.07 | 101.2 | 0.04 |


| [1] |
He, Y.; Sun, X.; Yan, X.; Li, Y.; Zhong, K.; Tang, L. J. Mater. Chem. C 2022, 10, 9009.
doi: 10.1039/D2TC00727D |
| [2] |
Driscoll, C. T.; Mason, R. P.; Chan, H. M.; Jacob, D. J.; Pirrone, N. Environ. Sci. Technol. 2013, 47, 4967.
doi: 10.1021/es305071v pmid: 23590191 |
| [3] |
Sarkar, A.; Chakraborty, S.; Lohar, S.; Ahmmed, E.; Saha, N. C.; Mandal, S. K.; Dhara, K.; Chattopadhyay, P. Chem. Res. Toxicol. 2019, 32, 1144.
doi: 10.1021/acs.chemrestox.9b00005 pmid: 30931555 |
| [4] |
Tang, A.; Chen, Z.; Deng, D.; Liu, G.; Tu, Y.; Pu, S. RSC Adv. 2019, 9, 11865.
doi: 10.1039/C9RA02119A |
| [5] |
Liu, X.; Liu, X.; Tao, M.; Zhang, W. J. Mater. Chem. A 2015, 3, 13254.
doi: 10.1039/C5TA02491A |
| [6] |
Luo, W.; Jiang, H.; Zhang, K.; Liu, W.; Tang, X.; Dou, W.; Ju, Z.; Li, Z.; Liu, W. J. Mater. Chem. B 2015, 3, 3459.
doi: 10.1039/C5TB00203F |
| [7] |
Gupta, S.; Milton, M. D. New J. Chem. 2018, 42, 2838.
doi: 10.1039/C7NJ04573E |
| [8] |
Fang, W.; Zhang, G.; Chen, J.; Kong, L.; Yang, L.; Bi, H.; Yang, J. Sens. Actuators, B 2016, 229, 338.
doi: 10.1016/j.snb.2016.01.130 |
| [9] |
Yang, L.; Su, Y.; Geng, Y.; Xiong, H.; Han, J.; Fang, Q.; Song, X. Org. Biomol. Chem. 2018, 16, 5036.
doi: 10.1039/c8ob00831k pmid: 29951681 |
| [10] |
Gao, T.; Huang, X.; Huang, S.; Dong, J.; Yuan, K.; Feng, X.; Liu, T.; Yu, K.; Zeng, W. J. Agric. Food. Chem. 2019, 67, 2377.
doi: 10.1021/acs.jafc.8b06895 |
| [11] |
Li, Y.; Chen, C.; Li, B.; Sun, J.; Wang, J.; Gao, Y.; Zhao, Y.; Chai, Z. J. Anal. At. Spectrom. 2006, 21, 94.
doi: 10.1039/B511367A |
| [12] |
Tseng, C. M.; De Diego, A.; Martin, F. M.; Amouroux, D.; Donard, O. F. X. J. Anal. At. Spectrom. 1997, 12, 743.
doi: 10.1039/a700956i |
| [13] |
Tian, M.; Wang, C.; Ma, Q.; Bai, Y.; Sun, J.; Ding, C. ACS Omega 2020, 5, 18176.
doi: 10.1021/acsomega.0c01790 |
| [14] |
Tang, L.; Yu, H.; Zhong, K.; Gao, X.; Li, J. RSC Adv. 2019, 9, 23316.
doi: 10.1039/C9RA04440J |
| [15] |
Zhong, K.; Yao, Y.; Sun, X.; Wang, Y.; Tang, L.; Li, X.; Zhang, J.; Yan, X.; Li, J. J. Agric. Food. Chem. 2022, 70, 5159.
doi: 10.1021/acs.jafc.2c00820 |
| [16] |
Wu, D.; Chen, L.; Lee, W.; Ko, G.; Yin, J.; Yoon, J. Coord. Chem. Rev. 2018, 354, 74.
doi: 10.1016/j.ccr.2017.06.011 |
| [17] |
Zhong, K.; Hu, X.; Zhou, S.; Liu, X.; Gao, X.; Tang, L.; Yan, X. J. Agric. Food. Chem. 2021, 69, 4628.
doi: 10.1021/acs.jafc.1c00862 |
| [18] |
Pan, Y.; Ban, L.; Li, J.; Liu, M.; Tang, L.; Yan, X. Dyes Pigm. 2022, 203, 110305.
doi: 10.1016/j.dyepig.2022.110305 |
| [19] |
Arivazhagan, C.; Borthakur, R.; Ghosh, S. Organometallics 2015, 34, 1147.
doi: 10.1021/om500948c |
| [20] |
Xu, D.; Tang, L.; Tian, M.; He, P.; Yan, X. Tetrahedron Lett. 2017, 58, 3654.
doi: 10.1016/j.tetlet.2017.08.016 |
| [21] |
Li, G.; Gao, G.; Cheng, J.; Chen, X.; Zhao, Y.; Ye, Y. Luminescence 2016, 31, 992.
doi: 10.1002/bio.3063 |
| [22] |
Guo, Y.; An, J.; Tang, H.; Peng, M.; Suzenet, F. Mater. Res. Bull. 2015, 63, 155.
doi: 10.1016/j.materresbull.2014.12.015 |
| [23] |
Hu, D.; Liao, S.; Chen, X.; Du, J.; Dawood, K.; Chauhan, S.; Gao, C.; Li, W. Bull. Korean Chem. Soc. 2020, 41, 686.
doi: 10.1002/bkcs.12054 |
| [24] |
Wang, J.; Niu, Q.; Hu, T.; Li, T.; Wei, T. J. Photochem. Photobiol., A 2019, 384, 112036.
doi: 10.1016/j.jphotochem.2019.112036 |
| [25] |
Tang, L.; Zhou, L.; Yan, X.; Zhong, K.; Gao, X.; Li, J. J. Photochem. Photobiol., A 2020, 387, 112160.
doi: 10.1016/j.jphotochem.2019.112160 |
| [26] |
Huang, L.; Yang, Z.; Zhou, Z.; Li, Y.; Tang, S.; Xiao, W.; Hu, M.; Peng, C.; Chen, Y.; Gu, B.; Li, H. Dyes Pigm. 2019, 163, 118.
doi: 10.1016/j.dyepig.2018.11.047 |
| [27] |
Liu, Y.-Y.; Naha, S.; Thirumalaivasan, N.; Velmathi, S.; Wu, S.-P. Sens. Actuators, B 2018, 277, 673.
doi: 10.1016/j.snb.2018.09.015 |
| [28] |
Gao, Y.; Ma, T.; Ou, Z.; Cai, W.; Yang, G.; Li, Y.; Xu, M.; Li, Q. Talanta 2018, 178, 663.
doi: 10.1016/j.talanta.2017.09.089 |
| [29] |
Huang, S.; Gao, T.; Bi, A.; Cao, X.; Feng, B.; Liu, M.; Du, T.; Feng, X.; Zeng, W. Dyes Pigm. 2020, 172, 107830.
doi: 10.1016/j.dyepig.2019.107830 |
| [30] |
Hong, Y.; Lam, J. W. Y.; Tang, B. Z. Chem. Commun. 2009, 4332.
|
| [31] |
Hong, Y.; Lam, J. W.; Tang, B. Z. Chem. Soc. Rev. 2011, 40, 5361.
doi: 10.1039/c1cs15113d |
| [32] |
Ozturk, S.; Atilgan, S. Tetrahedron Lett. 2014, 55, 70.
doi: 10.1016/j.tetlet.2013.10.105 |
| [33] |
Xu, D.; Tang, L.; Tian, M.; He, P.; Yan, X. Tetrahedron Lett. 2017, 58, 3654.
doi: 10.1016/j.tetlet.2017.08.016 |
| [34] |
Hu, D.; Liao, S.; Chen, X.; Du, J.; Dawood, K.; Chauhan, S.; Gao, C.; Li, W. Bull. Korean Chem. Soc. 2020, 41, 686.
doi: 10.1002/bkcs.12054 |
| [35] |
Liu, B.; Liu, J.; He, J.; Zhang, J.; Zhou, H.; Gao, C. Chem. Phys. 2020, 539, 110944.
doi: 10.1016/j.chemphys.2020.110944 |
| [1] | Shan Chen, Zhilin Chen, Qiong Hu, Yanshuang Meng, Yue Huang, Pingfang Tao, Liru Lu, Guobao Huang. Recognition of Bis-thiourea Tweezers to Neutral Molecules in Non-Polar Solvent [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 277-281. |
| [2] | Yingzhen Zhang, Dandan Jiang, Juanhua Li, Jingjing Wang, Kunming Liu, Jinbiao Liu. Construction Strategy and Imaging of Highly Selective Selenocysteine Fluorescent Probes [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 41-53. |
| [3] | Chongyang Zeng, Ping Hu, Biqin Wang, Wenyan Fang, Keqing Zhao. Cyanostilbene Bridged Triphenylene Dyad Stimuli-Responsive Discotic Liquid Crystal: Synthesis, Properties and Applications [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3287-3296. |
| [4] | Huanqing Li, Zhaohua Chen, Zujia Chen, Qiwen Qiu, Youcai Zhang, Sihong Chen, Zhaoyang Wang. Research Progress in Mercury Ion Fluorescence Probes Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3067-3077. |
| [5] | Binghui Ding, Shaohui Han, Haiqing Xiong, Benhua Wang, Bojun Zuo, Xiangzhi Song. A Highly Selective Ratiometric Fluorescent Probe for the Detection of Hypochlorite in Acute Lung Injury [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2878-2884. |
| [6] | Yang Zhao, Panpan Chen, Lizhi Han, Enju Wang. Aggregation-Induced Emission and Cell Imaging of Triphenylimidazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2454-2461. |
| [7] | Tiantian Liu, Hongpeng Zhang, Xiaomeng Jiao, Yinjuan Bai. Research Progress of Multi-signal Fluorescent Probes for Simultaneous Detection of Biothiols [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2081-2095. |
| [8] | Feiran Liu, Jing Jing, Xiaoling Zhang. Research Progress of Fluorescent Probes for Cysteine Targeting Cellular Organelles [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2053-2067. |
| [9] | Yifang Li, Yao Wang, Huawei Niu, Xiujin Chen, Zhaozhou Li, Yongguo Wang. Research Progress of Sulfur Dioxide Fluorescent Probe Targeting Mitochondria [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1952-1962. |
| [10] | Zhihua Chen, Yan Hu, Lili Ma, Ziyi Zhang, Chuanxiang Liu. Rational Design of ortho-Vinylhydropyridine-Assisted Amino-fluorophore as Hypochlorite Fluorescent Probe [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 718-724. |
| [11] | Hongwei Tang, Chao Wang, Keli Zhong, Shuhua Hou, Lijun Tang, Yanjiang Bian. A Naked-Eye and Fluorescent Dual-Channel Probe for Rapid Detection of Hg2+ and Its Multiple Applications [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 712-717. |
| [12] | Huiming Lu, Lamaocao Ma, Hengchang Ma. Research Progress and Prospect of Aggregation-Induced Emission Supramolecular Luminescence Materials [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4075-4105. |
| [13] | Yuehua Zhang, Fei Nie, Lu Zhou, Xiaofeng Wang, Yuan Liu, Yanping Huo, Wencheng Chen, Zujin Zhao. Synthesis and Optoelectronic Studies of Thermally Activated Delayed Fluorescence Materials Based on Benzothiazolyl Ketones [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3876-3887. |
| [14] | Yanhui Ma, Yuqian Wu, Xiaoxu Wang, Gui Gao, Xin Zhou. Research Progress of Near-Infrared Fluorescent Probes Based on 1,3-Dichloro-7-hydroxy-9,9-dimethyl-2(9H)-acridone (DDAO) [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 94-111. |
| [15] | Yaxin Yang, Lin Chen, Xiaoling Hu, Keli Zhong, Shidi Li, Xiaomei Yan, Jinglin Zhang, Lijun Tang. Synthesis of a Turn-On Fluorescent Probe for Hydrogen Sulfide and Its Application in Red Wine and Living Cells [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 308-312. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||