Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (11): 3876-3887.DOI: 10.6023/cjoc202303022 Previous Articles Next Articles
张越华a,c,d, 聂飞c,d, 周路c,d, 王晓烽c,d, 刘源c,d,*( ), 霍延平c,e, 陈文铖c,d,*(
), 霍延平c,e, 陈文铖c,d,*( ), 赵祖金b,*(
), 赵祖金b,*( )
)
收稿日期:2023-03-15
修回日期:2023-05-30
发布日期:2023-07-12
基金资助:
Yuehua Zhanga,c,d, Fei Niec,d, Lu Zhouc,d, Xiaofeng Wangc,d, Yuan Liuc,d( ), Yanping Huoc,e, Wencheng Chenc,d(
), Yanping Huoc,e, Wencheng Chenc,d( ), Zujin Zhaob(
), Zujin Zhaob( )
)
Received:2023-03-15
Revised:2023-05-30
Published:2023-07-12
Contact:
E-mail: Supported by:Share
Yuehua Zhang, Fei Nie, Lu Zhou, Xiaofeng Wang, Yuan Liu, Yanping Huo, Wencheng Chen, Zujin Zhao. Synthesis and Optoelectronic Studies of Thermally Activated Delayed Fluorescence Materials Based on Benzothiazolyl Ketones[J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3876-3887.

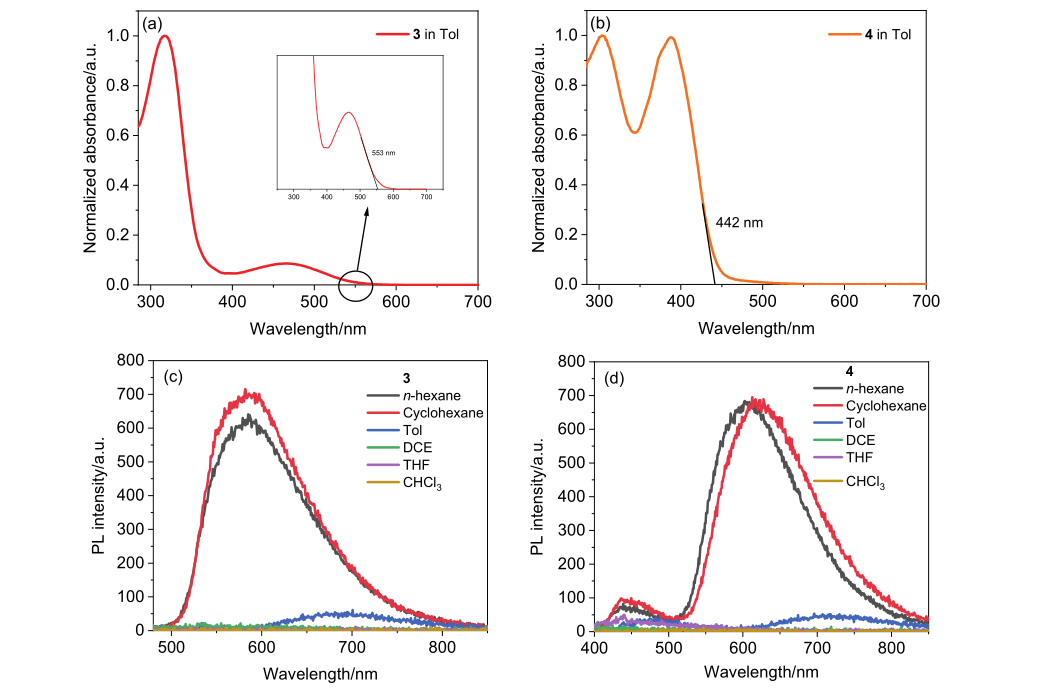
| Compd. | λabs/nm | λPL/nm | PLQY/% | τp/ns | τd/μs | Td/Tg | ES1/ET1 | EHOMO/ELUMO |
|---|---|---|---|---|---|---|---|---|
| 3 | 317/458a | 586a, 683b, 630c | 0.8b, 10.0c | 9.15c | 0.63c | 338/57 | 2.26/2.22c | –5.15/–2.91d |
| 4 | 304/389a | 440/605a, 654b, 609c | 3.6b, 45.9c | 21.8c | 1.30c | 351/66 | 2.69/2.53c | –5.14/–2.33d |
| Compd. | λabs/nm | λPL/nm | PLQY/% | τp/ns | τd/μs | Td/Tg | ES1/ET1 | EHOMO/ELUMO |
|---|---|---|---|---|---|---|---|---|
| 3 | 317/458a | 586a, 683b, 630c | 0.8b, 10.0c | 9.15c | 0.63c | 338/57 | 2.26/2.22c | –5.15/–2.91d |
| 4 | 304/389a | 440/605a, 654b, 609c | 3.6b, 45.9c | 21.8c | 1.30c | 351/66 | 2.69/2.53c | –5.14/–2.33d |

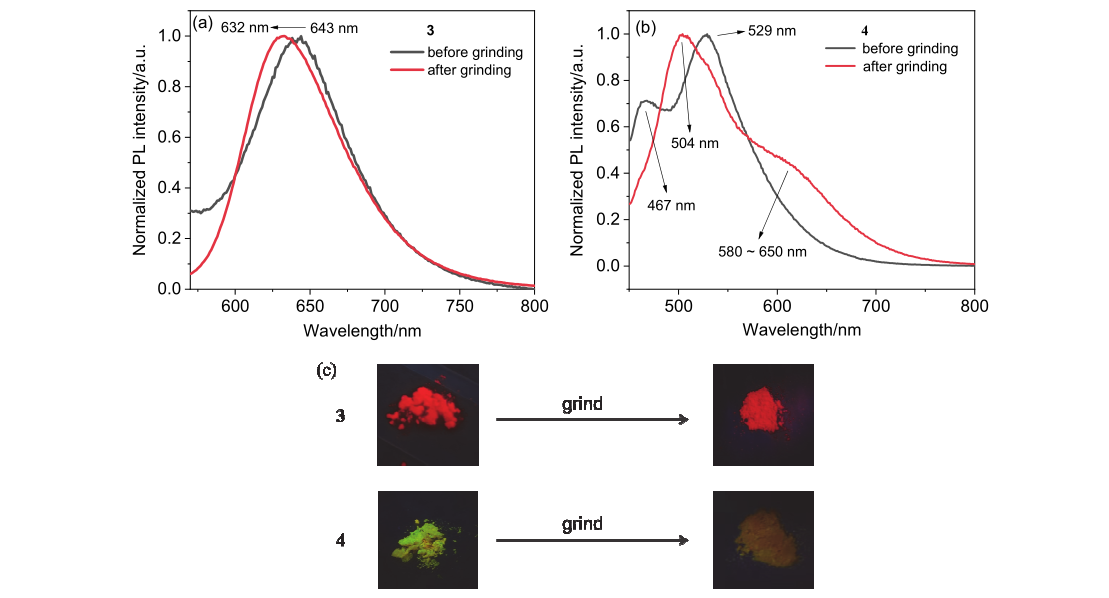
| Temperature/K | τp/ns | τd/μs | Ratio/% | |||||
|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 3 | 4 | 3 | 4 | |||
| 300 | 9.15 | 21.8 | 0.63 | 1.30 | 68/32 | 48/52 | ||
| 200 | 9.45 | 26.6 | 0.94 | 1.50 | 66/34 | 54/46 | ||
| 100 | 8.43 | 28.0 | 1.17 | 1.39 | 61/38 | 68/32 | ||
| Temperature/K | τp/ns | τd/μs | Ratio/% | |||||
|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 3 | 4 | 3 | 4 | |||
| 300 | 9.15 | 21.8 | 0.63 | 1.30 | 68/32 | 48/52 | ||
| 200 | 9.45 | 26.6 | 0.94 | 1.50 | 66/34 | 54/46 | ||
| 100 | 8.43 | 28.0 | 1.17 | 1.39 | 61/38 | 68/32 | ||
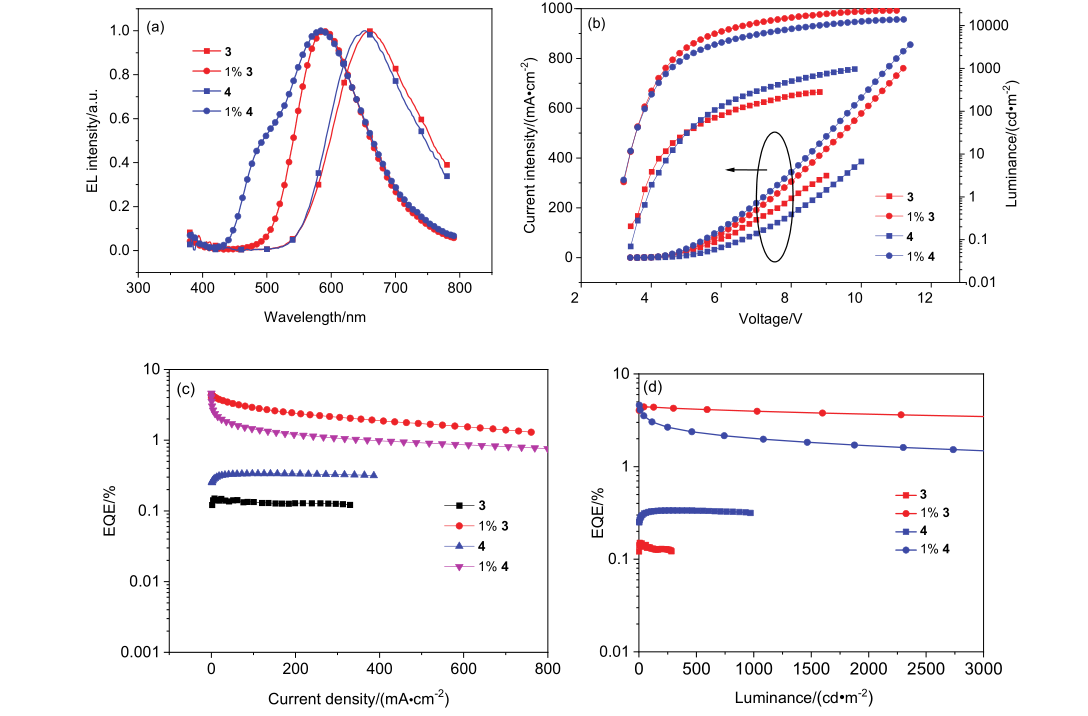
| Device | Von/V | Lmax/(cd•m–2) | Maximum value/the value at 1000 cd•m–2 | λEL/nm | CIE (x, y) | Roll-off of EQE/% | ||
|---|---|---|---|---|---|---|---|---|
| CE/(cd•A–1) | PE/(lm•W–1) | EQE/% | ||||||
| A | 4.0 | 283 | 0.11/— | 0.07/— | 0.15/— | 662 | (0.604, 0.364) | — |
| B | 3.8 | 2729 | 1.48/1.17 | 1.11/0.51 | 1.10/0.87 | 620 | (0.585, 0.412) | 20.9 |
| C | 3.8 | 971 | 3.58/— | 2.96/— | 2.15/— | 612 | (0.563, 0.432) | — |
| D | 3.8 | 1459 | 5.47/2.54 | 5.46/0.91 | 2.77/1.29 | 600 | (0.539, 0.454) | 53.4 |
| E | 3.4 | 26680 | 10.14/9.11 | 8.65/6.22 | 4.42/4.00 | 589 | (0.505, 0.480) | 9.5 |
| F | 4.2 | 971 | 0.20/— | 0.15/— | 0.34/— | 652 | (0.609, 0.380) | — |
| G | 3.6 | 5073 | 3.40/2.50 | 2.80/1.2 | 1.80/1.30 | 604 | (0.500, 0.455) | 27.8 |
| H | 3.8 | 1164 | 5.16/1.61 | 4.27/0.50 | 2.62/0.82 | 604 | (0.499, 0.456) | 68.7 |
| I | 3.6 | 1208 | 7.56/1.77 | 6.59/0.59 | 3.45/0.81 | 589 | (0.478, 0.463) | 76.5 |
| J | 3.4 | 13950 | 10.42/4.46 | 9.63/2.92 | 4.62/2.00 | 584 | (0.425, 0.461) | 56.7 |
| Device | Von/V | Lmax/(cd•m–2) | Maximum value/the value at 1000 cd•m–2 | λEL/nm | CIE (x, y) | Roll-off of EQE/% | ||
|---|---|---|---|---|---|---|---|---|
| CE/(cd•A–1) | PE/(lm•W–1) | EQE/% | ||||||
| A | 4.0 | 283 | 0.11/— | 0.07/— | 0.15/— | 662 | (0.604, 0.364) | — |
| B | 3.8 | 2729 | 1.48/1.17 | 1.11/0.51 | 1.10/0.87 | 620 | (0.585, 0.412) | 20.9 |
| C | 3.8 | 971 | 3.58/— | 2.96/— | 2.15/— | 612 | (0.563, 0.432) | — |
| D | 3.8 | 1459 | 5.47/2.54 | 5.46/0.91 | 2.77/1.29 | 600 | (0.539, 0.454) | 53.4 |
| E | 3.4 | 26680 | 10.14/9.11 | 8.65/6.22 | 4.42/4.00 | 589 | (0.505, 0.480) | 9.5 |
| F | 4.2 | 971 | 0.20/— | 0.15/— | 0.34/— | 652 | (0.609, 0.380) | — |
| G | 3.6 | 5073 | 3.40/2.50 | 2.80/1.2 | 1.80/1.30 | 604 | (0.500, 0.455) | 27.8 |
| H | 3.8 | 1164 | 5.16/1.61 | 4.27/0.50 | 2.62/0.82 | 604 | (0.499, 0.456) | 68.7 |
| I | 3.6 | 1208 | 7.56/1.77 | 6.59/0.59 | 3.45/0.81 | 589 | (0.478, 0.463) | 76.5 |
| J | 3.4 | 13950 | 10.42/4.46 | 9.63/2.92 | 4.62/2.00 | 584 | (0.425, 0.461) | 56.7 |
| [1] |
Zhou, J.; Tian, X. Y.; Wang, B. K.; Zhang, S. S.; Liu, Z. H.; Chen, W. Acta Chim. Sinica 2022, 80, 395. (in Chinese)
doi: 10.6023/A21110513 |
|
(周静, 田雪迎, 王斌凯, 张沙沙, 刘宗豪, 陈炜, 化学学报, 2022, 80, 395.)
doi: 10.6023/A21110513 |
|
| [2] |
Liang, Z. P.; Tang, R.; Qiu, Y. C.; Wang, Y.; Lu, H.; Wu, Z. G. Acta Chim. Sinica 2021, 79, 1401. (in Chinese)
doi: 10.6023/A21070355 |
|
(梁志鹏, 唐瑞, 邱雨晨, 王阳, 陆洪彬, 吴正光, 化学学报, 2021, 79, 1401.)
doi: 10.6023/A21070355 |
|
| [3] |
Sun, N.; Jiang, C.; Li, Q.; Tan, D.; Bi, S.; Song, J. J. Mater. Sci.: Mater. Electron. 2020, 31, 20688.
|
| [4] |
Yang, X.; Jiao, B.; Dang, J. S.; Sun, Y.; Wu, Y.; Zhou, G.; Wong, W. Y. ACS Appl. Mater. Interfaces 2018, 10, 10227.
doi: 10.1021/acsami.7b18330 |
| [5] |
Wong, M. Y.; Zysman-Colman, E. Adv. Mater. 2017, 29, 1605444.
doi: 10.1002/adma.v29.22 |
| [6] |
Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H. H.; Chen, R. F.; Zheng, C.; Zhang, L.; Huang, W. Adv. Mater. 2014, 26: 7931.
doi: 10.1002/adma.v26.47 |
| [7] |
Di, B. H.; Chen, Y. L. Chin. Chem. Lett. 2018, 29, 245.
doi: 10.1016/j.cclet.2017.08.043 |
| [8] |
Huang, C.; Qiu, Z. P.; Gao, Y.; Chen, W. C.; Ji, S. M.; Huo, Y. P. Chin. J. Org. Chem. 2021, 41, 3050. (in Chinese)
|
|
(黄酬, 邱志鹏, 高杨, 陈文铖, 籍少敏, 霍延平, 有机化学, 2021, 41, 3050.)
doi: 10.6023/cjoc202101053 |
|
| [9] |
Tan, J. H.; Huo, Y. P.; Cai, N.; Ji, S. M.; Li, Z. Z.; Zhang, L. Chin. J. Org. Chem. 2017, 37, 2457. (in Chinese)
|
|
(谭继华, 霍延平, 蔡宁, 籍少敏, 李宗植, 张力, 有机化学, 2017, 37, 2457.)
doi: 10.6023/cjoc201704015 |
|
| [10] |
Sarada, G.; Cho, W.; Maheshwaran, A.; Sree, V. G.; Park, H. Y.; Gal, Y. S.; Song, M.; Jin, S. H. Adv. Funct. Mater. 2017, 27, 1701002.
doi: 10.1002/adfm.v27.27 |
| [11] |
Zheng, Y. T.; Zuo, L. Q.; Zhang, L. T.; Huang, Z. H.; Li, S. F.; Yang, Z.; Mao, Z.; Luo, S. L.; Liu, C.; Sun, F. Q.; Shi, G.; Chi, Z. G.; Xu, B. J. Chin. Chem. Lett. 2022, 33, 4536.
doi: 10.1016/j.cclet.2022.01.059 |
| [12] |
Cao, H. T.; Hou, P. F.; Cao, Q.; Li, Y. A.; Wang, S. S.; Xie, L. H. Acta Chim. Sinica 2022, 80, 1476. (in Chinese)
doi: 10.6023/A22070335 |
|
(曹洪涛, 侯鹏飞, 曹庆, 李延昂, 汪莎莎, 解令海, 化学学报, 2022, 80, 1476.)
doi: 10.6023/A22070335 |
|
| [13] |
Shi, Q.; Wang, L. Y. Chin. J. Org. Chem. 2022, 42, 1256. (in Chinese)
doi: 10.6023/cjoc202200019 |
|
(石强, 王乐勇, 有机化学, 2022, 42, 1256.)
doi: 10.6023/cjoc202200019 |
|
| [14] |
Guo, J. J.; Zhao, Z. J.; Tang, B. Z. Adv. Opt. Mater. 2018, 6, 1800264.
doi: 10.1002/adom.v6.15 |
| [15] |
Sagara, Y.; Shizu, K.; Tanaka, H.; Miyazaki, H.; Goushi, K.; Kaji, H.; Adachi, C. Chem. Lett. 2015, 44, 360.
doi: 10.1246/cl.141054 |
| [16] |
Ahn, D. H.; Kim, S. W.; Lee, H.; Ko, I. J.; Karthik, D.; Lee, J. Y.; Kwon, J. H. Nat. Photonics 2019, 13, 540.
doi: 10.1038/s41566-019-0415-5 |
| [17] |
Wu, T. L.; Huang, M. J.; Lin, C. C.; Huang, P. Y.; Chou, T. Y.; Chen-Cheng, R. W.; Lin, H. W.; Liu, R. S.; Cheng, C. H. Nat. Photonics 2018, 12, 235.
doi: 10.1038/s41566-018-0112-9 |
| [18] |
Bryden, M. A.; Zysman-Colman, E. Chem. Soc. Rev. 2021, 50, 7587.
doi: 10.1039/D1CS00198A |
| [19] |
Zhang, T.; Zhou, Z.; Liu, X.; Wang, K.; Fan, Y.; Zhang, C.; Yao, J.; Yan, Y.; Zhao, Y. S. J. Am. Chem. Soc. 2021, 143, 20249.
doi: 10.1021/jacs.1c08824 |
| [20] |
Steinegger, A.; Klimant, I.; Borisov, S. M. Adv. Opt. Mater. 2017, 5, 1700372.
doi: 10.1002/adom.v5.18 |
| [21] |
Wong, M. Y.; Zysman-Colman, E. Adv. Mater. 2017, 29, 1605444.
doi: 10.1002/adma.v29.22 |
| [22] |
Fang, F.; Yuan, Y.; Wan, Y.; Li, J.; Song, Y.; Chen, W.; Zhao, D.; Chi, Y.; Li, M.; Lee, C. Small 2022, 18, 2106215.
doi: 10.1002/smll.v18.6 |
| [23] |
Fang, F.; Zhu, L.; Li, M.; Song, Y. Y.; Sun, M.; Zhao, D. G.; Zhang, J. F. Adv. Sci. 2021, 8, 2102970.
doi: 10.1002/advs.v8.24 |
| [24] |
Tan, J. M.; Yu, Y. J.; Guan, M.; Zhao, Y. H.; Tang, Z. L.; Zhou, Z. H.; Guo, T. Chin. J. Org. Chem. 2022, 42, 3776. (in Chinese)
doi: 10.6023/cjoc202204038 |
|
(谭佳敏, 余雅君, 关猛, 赵云辉, 唐子龙, 周智华, 郭涛, 有机化学, 2022, 42, 3776.)
doi: 10.6023/cjoc202204038 |
|
| [25] |
Zeng, W.; Lin, M. H.; Zhu, L. Y.; Lin, M. J. Chin. J. Chem. 2022, 40, 39.
doi: 10.1002/cjoc.v40.1 |
| [26] |
Ma, H.; Peng, Q.; An, Z.; Huang, W.; Shuai, Z. J. Am. Chem. Soc. 2018, 141, 1010.
doi: 10.1021/jacs.8b11224 |
| [27] |
Ma, R.; Ding, Y.; Chen, R.; Wang, Z.; Ma, Y. J. Org. Chem. 2020, 86, 310.
doi: 10.1021/acs.joc.0c02095 |
| [28] |
Okazaki, M.; Takeda, Y.; Data, P.; Pander, P.; Higginbotham, H.; Monkman, A. P.; Minakata, S. Chem. Sci. 2017, 8, 2677.
doi: 10.1039/c6sc04863c pmid: 28553504 |
| [29] |
Huang, B.; Chen, W. C.; Li, Z. J.; Zhang, J. F.; Zhao, W. J.; Feng, Y.; Tang, B. Z.; Lee, C. S. Angew. Chem., Int. Ed. 2018, 57, 12473.
doi: 10.1002/anie.v57.38 |
| [30] |
Xu, B. J.; Mu, Y. X.; Mao, Z.; Xie, Z. L.; Wu, H. Z.; Zhang, Y.; Jin, C. J.; Chi, Z. G.; Liu, S. W.; Xu, J. R.; Wu, Y. C.; Lu, P. Y.; Lien, A.; Bryce, M. R. Chem. Sci. 2016, 7, 2201.
doi: 10.1039/C5SC04155D |
| [31] |
Han, M.; Chen, Y.; Xie, Y.; Zhang, F.; Li, Z. Cell Rep. Phys. Sci. 2020, 1, 100252.
|
| [32] |
Borowicz, P.; Herbich, J.; Kapturkiewicz, A.; Opallo, M.; Nowacki, J. Chem. Phys. 1999, 249, 49.
doi: 10.1016/S0301-0104(99)00265-7 |
| [33] |
Li, S. S.; Huang, X.; Gao, Y. L.; Jin, J. Org. Lett. 2022, 24, 5817.
doi: 10.1021/acs.orglett.2c02364 |
| [34] |
Acar, N.; Kurzawa, J.; Fritz, N.; Stockmann, A.; Roman, C.; Schneider, S.; Clark, T. J. Phys. Chem. A 2003, 107, 9530.
doi: 10.1021/jp036250u |
| [35] |
Polgar, A. M.; Poisson, J.; Paisley, N. R.; Christopherson, C. J.; Reyes, A. C.; Hudson, Z. M. Macromolecules 2020, 53, 2039.
doi: 10.1021/acs.macromol.0c00287 |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||