Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (5): 1649-1657.DOI: 10.6023/cjoc202309009 Previous Articles Next Articles
ARTICLES
收稿日期:2023-09-11
修回日期:2023-11-12
发布日期:2024-01-11
基金资助:
Yihong Wang, Wenli Li, Hailu Lin, Zhanggao Le( ), Zongbo Xie(
), Zongbo Xie( )
)
Received:2023-09-11
Revised:2023-11-12
Published:2024-01-11
Contact:
*E-mail: Supported by:Share
Yihong Wang, Wenli Li, Hailu Lin, Zhanggao Le, Zongbo Xie. Reaction of 2-Aminobenzyl Alcohols with β-Dicarbonyl Compounds to Synthesize Quinoline Derivatives in Water Promoted by Active Manganese Dioxide[J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1649-1657.
| Entry | MnO2 | Solvent | n(1a)∶n(2a) | Time/h | Yieldb/% |
|---|---|---|---|---|---|
| 1 | 3.0 | H2O | 1.5∶1.0 | 24 | 67 |
| 2 | 3.0 | 1,4-Dioxane | 1.5∶1.0 | 24 | Trace |
| 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 | 3.0 3.0 3.0 3.0 — 0.5 1.0 1.5 2.0 2.5 3.5 4.0 3.5 3.5 3.5 3.5 3.5 3.5 3.5 3.5 3.5 3.5 3.5 3.5 | EtOH CH3CN CH2Cl2 DMSO H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O | 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.0∶2.0 1.0∶1.5 1.0∶1.0 1.5∶1.0 2.0∶1.0 3.0∶1.0 2.0∶1.0 2.0∶1.0 2.0∶1.0 2.0∶1.0 2.0∶1.0 2.0∶1.0 | 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 10 12 14 16 18 20 | 18 Trace Trace None Trace 16 27 47 50 64 75 78 54 64 65 72 80 83 56 64 70 74 77 78 |
| Entry | MnO2 | Solvent | n(1a)∶n(2a) | Time/h | Yieldb/% |
|---|---|---|---|---|---|
| 1 | 3.0 | H2O | 1.5∶1.0 | 24 | 67 |
| 2 | 3.0 | 1,4-Dioxane | 1.5∶1.0 | 24 | Trace |
| 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 | 3.0 3.0 3.0 3.0 — 0.5 1.0 1.5 2.0 2.5 3.5 4.0 3.5 3.5 3.5 3.5 3.5 3.5 3.5 3.5 3.5 3.5 3.5 3.5 | EtOH CH3CN CH2Cl2 DMSO H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O H2O | 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.5∶1.0 1.0∶2.0 1.0∶1.5 1.0∶1.0 1.5∶1.0 2.0∶1.0 3.0∶1.0 2.0∶1.0 2.0∶1.0 2.0∶1.0 2.0∶1.0 2.0∶1.0 2.0∶1.0 | 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 10 12 14 16 18 20 | 18 Trace Trace None Trace 16 27 47 50 64 75 78 54 64 65 72 80 83 56 64 70 74 77 78 |
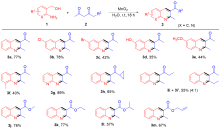
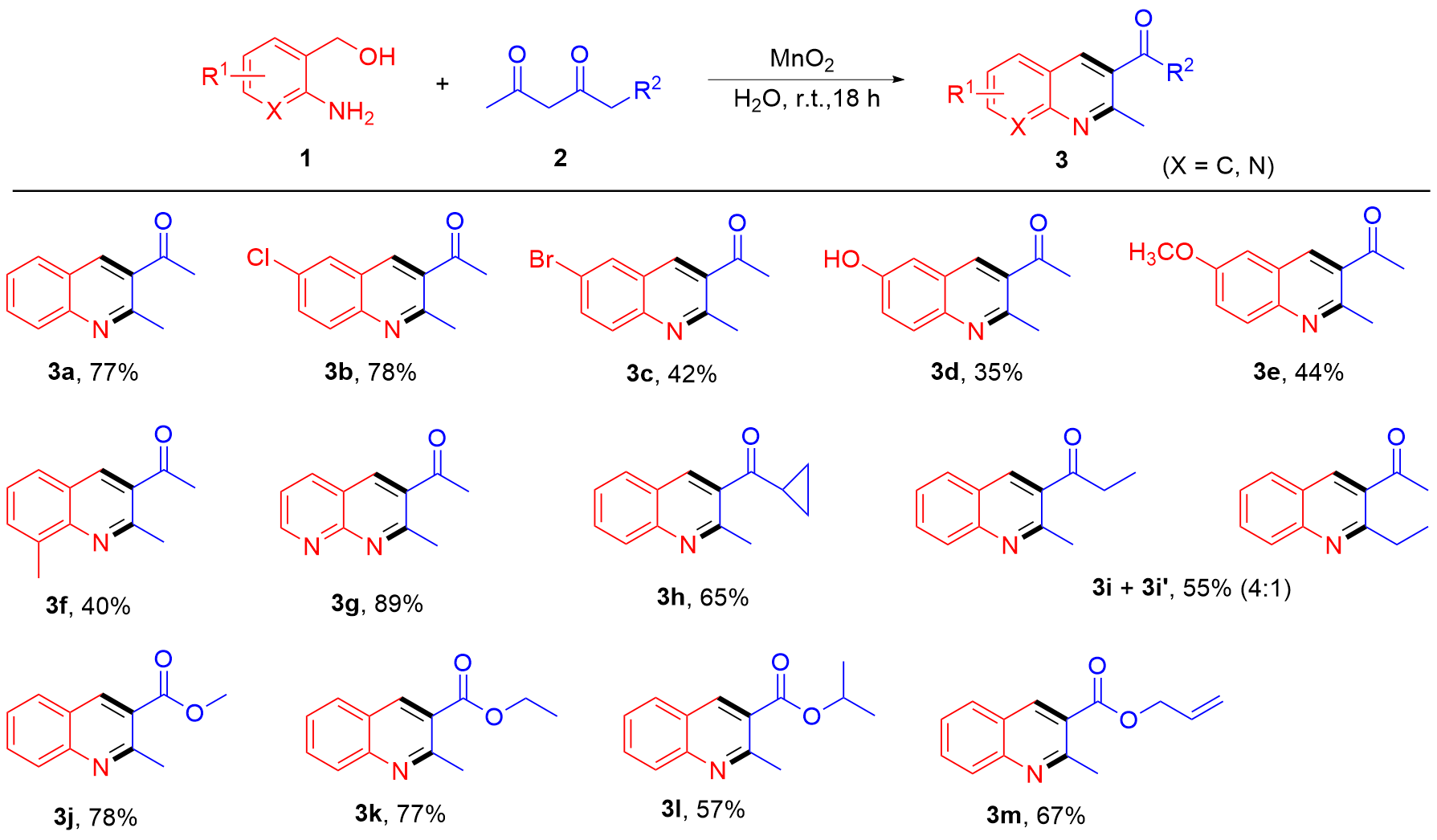
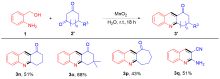
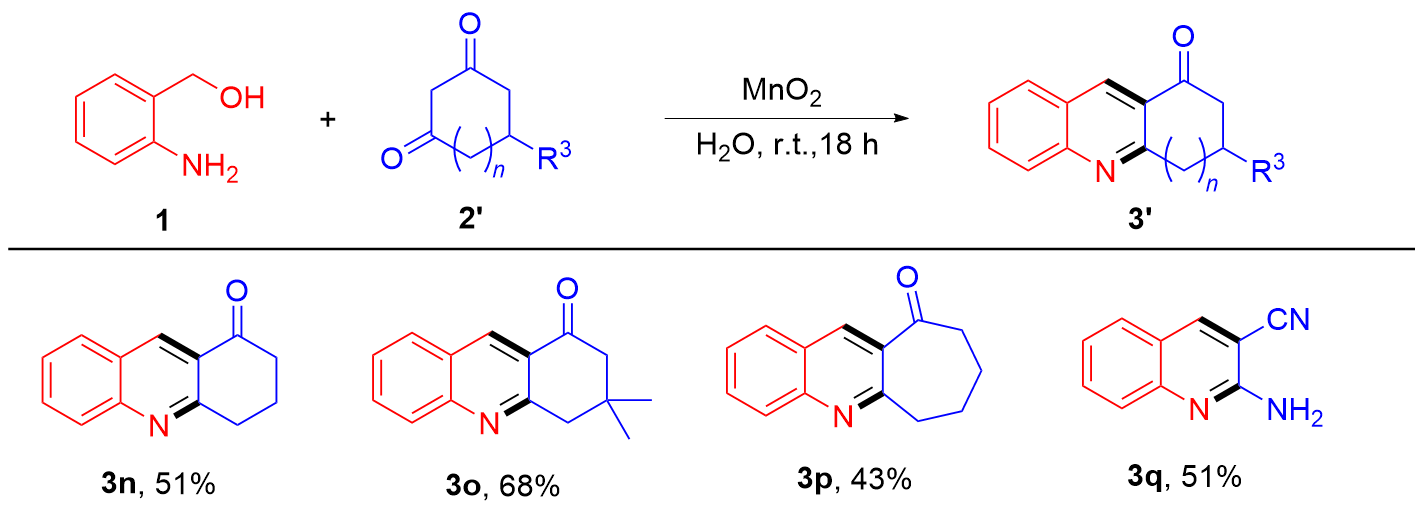
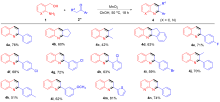

| [1] |
Boyd, D. R.; Sharma, N. D.; Loke, P. L.; Malone, J. F.; McRoberts, W. C.; Hamilton, J. T. Org. Biomol. Chem. 2007, 5, 2983.
|
| [2] |
(a) Sun, N.; Du, R. L.; Zheng, Y. Y.; Huang, B. H.; Guo, Q.; Zhang, R. F.; Wong, K. Y.; Lu, Y. J. Eur. J. Med. Chem. 2017, 135, 1.
pmid: 27983846 |
|
(b) Zablotskaya, A.; Segal, I.; Geronikaki, A.; Shestakova, I.; Nikolajeva, V.; Makarenkova, G. Pharmacol. Rep. 2017, 69, 575.
doi: S1734-1140(16)30130-X pmid: 27983846 |
|
|
(c) Nathubhai, A.; Haikarainen, T.; Koivunen, J.; Murthy, S.; Koumanov, F.; Lloyd, M. D.; Holman, G. D.; Pihlajaniemi, T.; Tosh, D.; Lehti, L. J. Med. Chem. 2017, 60, 814.
doi: 10.1021/acs.jmedchem.6b01574 pmid: 27983846 |
|
|
(d) Murugavel, S.; Stephen, C. S. J. P.; Subashini, R.; AnanthaKrishnan, D. J. Photochem. Photobiol. B 2017, 173, 216.
pmid: 27983846 |
|
|
(e) Keri, R. S.; Patil, S. A. Biomed. Pharmacother. 2014, 68, 1161.
pmid: 27983846 |
|
|
(f) García, E.; Coa, J. C.; Otero, E.; Carda, M.; Vélez, I. D.; Robledo, S. M.; Cardona, W. I. Med. Chem. Res. 2018, 27, 497.
pmid: 27983846 |
|
|
(g) Fouda, A. M. Med. Chem. Res. 2017, 26, 302.
pmid: 27983846 |
|
|
(h) Pinz, M. P.; Reis, A. S.; Leivas, R.; Voss, G. T.; Wilhelm, E. A. Regul. Toxicol. Pharm. 2017, 90, 72.
pmid: 27983846 |
|
| [3] |
(a) Aly, M.; Ibrahim, M. M.; Okael, A.; Gherbawy, Y. Russ. J. Bioorg. Chem. 2014, 40, 214.
|
|
(b) Chen, R.; Yang, X.; Tian, H.; Sun, L. J. Photochem. Photobiol. A: Chem. 2007, 189, 295.
|
|
|
(c) Song, S.; Zhao, W.; Wang, L.; Redshaw, C.; Wang, F.; Sun, W. J. Organomet. Chem. 2011, 696, 3029.
|
|
| [4] |
(a) Denmark, S. E.; Venkatraman, S. J. Org. Chem. 2006, 71, 1668.
pmid: 16468822 |
|
(b) Waldvogel, S. R. Adv. Synth. Catal. 2004, 345, 91.
pmid: 16468822 |
|
|
(c) Li, X.; Mao, Z.; Wang, Y.; Chen, W.; Lin, X. Tetrahedron 2011, 67, 3858.
pmid: 16468822 |
|
|
(d) Wang, L. M.; Hu, L.; Chen, H. J.; Sui, Y. Y.; Shen, W. ChemInform 2009, 40, 406.
pmid: 16468822 |
|
|
(e) Brouet, J. C.; Gu, S.; Peet, N. P.; Williams, J. D. ChemInform 2009, 39, 1563.
pmid: 16468822 |
|
|
(f) Bharate, J. B.; Vishwakarma, R. A.; Bharate, S. B. Rsc. Adv. 2015, 5, 42020.
pmid: 16468822 |
|
|
(g) Cadamuro, B. S. Tetrahedron Lett. 2010, 51, 2342.
pmid: 16468822 |
|
| [5] |
Wang, L.; Shen, Q.; Yu, J. J.; Liu, M. T.; Qiu, J.; Fang, L.; Guo, F. L.; Tang, J. Synthesis 2012, 44, 389.
|
| [6] |
Marco-Contelles, J.; Pérez-Mayoral, E.; Samadi, A.; Carreiras, M. C.; Soriano, E. Chem. Rev. 2009, 109, 2652.
doi: 10.1021/cr800482c pmid: 19361199 |
| [7] |
(a) Singh, V. K.; Donthireddy, S. N. R.; Pandey, V. K.; Rit, A. Org. Biomol. Chem. 2022, 20, 1945.
pmid: 30047973 |
|
(b) Sundarraman, B.; Rengan, R.; Semeril, D. Organometallics 2022, 41, 1314.
pmid: 30047973 |
|
|
(c) Xiong, B.; Wang, Y. Y.; Liu, Y.; Bao, Y. D.; Liu, Z. G.; Zhang, Y. N.; Liu, Y. Org. Biomol. Chem. 2018, 16, 5707.
doi: 10.1039/c8ob01321g pmid: 30047973 |
|
|
(d) Xu, J. X.; Chen, Q. M.; Luo, Z. G.; Tang, X. D.; Zhao, J. W. RSC Adv. 2019, 9, 28764.
pmid: 30047973 |
|
|
(e) Sk, M.; Bera, A.; Banerjee, D. ChemCatChem 2023, 15, 1.
pmid: 30047973 |
|
| [8] |
Cini, E.; Petricci, E.; Truglio, G. I.; Vecchio, M.; Taddei, M. ChemInform 2016, 47, 31386.
|
| [9] |
Liu, X.; Liu, X.; Bi, Y.; Ke, J. Energ. Fuel. 2022, 36, 13238.
|
| [10] |
Chen, W. D.; Wu, X. Adsorpt. Sci. Technol. 2018, 36, 3.
|
| [11] |
Varma, R. S.; Saini, R. K.; Dahiya, R. Tetrahedron Lett. 1997, 38, 7823.
|
| [12] |
Lu, J. Q.; Zeng, J.; Abulikemu, A. R. Chin. J. Org. Chem. 2020, 40, 2483 (in Chinese).
|
|
( 吕进强, 曾竟, 阿布都热西提•阿布力克木, 有机化学, 2020, 40, 2483.)
|
|
| [13] |
Pant, P. L.; Sonune, R. K.; Shankarling, G. S. ChemistrySelect 2018, 3, 5249.
|
| [14] |
Kalla, R. M. N.; Zhang, Y.; Kim, Ⅱ. New J. Chem. 2017, 41, 5373.
|
| [15] |
Anand, N.; Koley, S.; Ramulu, B. J.; Singh, M. S. Org. Biomol. Chem. 2015, 13, 9570.
|
| [16] |
Choudhury, S. S.; Jena, S.; Sahoo, D. K.; Shekh, S.; Kar, R. K.; Dhakad, A.; Gowd, K. H.; Biswal, H. S. ACS Omega 2021, 6, 19304.
|
| [17] |
Rajawinslin, R. R.; Gawande, S. D.; Kavala, V.; Huang, Y. H.; Kuo, C. W.; Kuo, T. S.; Chen, M. L.; He, C. H.; Yao, C. F. RSC Adv. 2014, 4, 37806.
|
| [18] |
Marina, G. O.; Antonio, J. L. P.; Rosa, M. M. A.; Jacek, P.; Elena, P. M.; Elena, S. ChemCatChem 2014, 6, 3440.
|
| [19] |
Sergey, V.; Elena, V. V.; Konstantin, L. Ivanov.; Ekaterina, M. B.; Mikhail, Y. M. Eur. J. Org. Chem. 2017, 19, 2814.
|
| [20] |
Kaewmee, B.; Rukachaisirikul, V.; Kaeobamrung, J. Org. Biomol. Chem. 2017, 15, 7387
|
| [21] |
Anderson, E. C.; Sneddon, H. F.; Hayes, C. J. Green Chem. 2019, 21, 3050.
doi: 10.1039/c9gc00408d |
| [22] |
Kishore, P. S.; Gujjarappa, R.; Putta, R. K.; Polina, S.; Singh, V.; Malakar, C. C.; Pujar, P. P. ChemistrySelect 2022, 7, 1.
|
| [23] |
Zhang, S. L.; Deng, Z. Q. Org. Biomol. Chem. 2016, 14, 8966.
|
| [24] |
Qu, F.; He, P.; Hu, R. F.; Cheng, X. H.; Wang, S.; Wu, J. Synth. Commun. 2015, 45, 2802.
|
| [25] |
Xu, J. X.; Pan, N. L.; Chen, J. X.; Zhao, J. W. J. Org. Chem. 2021, 86, 10747.
|
| [26] |
Cao, F.; Mao, A. R.; Yang, B. B.; Ge, C. Y.; Wang, D. W. New J. Chem. 2021, 45, 6768.
|
| [1] | Changjun Liu, Huiling Hu, Chenghong Liu, Chaojie Zhu, Tiandi Tang. Pd Supported on Mesoporous ETS-10 Zeolite Catalyst with Superior Catalytic Performances in Synthesizing 1,2-Diones from the Oxidation of Internal Alkynes [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2953-2960. |
| [2] | Wenqian Wu, Chunxia Chen, Jinsong Peng, Zhanyu Li. Research Progress of Carbonyl α-Position Amination [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2743-2763. |
| [3] | Xiantao Ma, Xiaoyu Yan, Yingying Zhu, Shuanglin Niu, Yuxuan Wang, Chao Yuan. Water-Promoted Green Synthesis of Heteroaryl Thioether [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2136-2142. |
| [4] | Wenting Wei, Zhuangzhuang Li, Wandi Li, Jiaqi Li, Xianying Shi. Green Method for Constructing Phthalides via Oxidative Coupling of Aromatic Acids and Acrylates in Neat Water and Air [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1177-1186. |
| [5] | Peng Zhou, Weiming Zhu, Jiantao Zhang, Duoduo Xiao, Xiangfeng Guo, Weibing Liu. Cobalt-Catalyzed Oxyalkylation Reaction of Styrenes: Rapid Access to α-Alkyl Substituted Acetophenone Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3939-3944. |
| [6] | Yuehua Zhang, Fei Nie, Lu Zhou, Xiaofeng Wang, Yuan Liu, Yanping Huo, Wencheng Chen, Zujin Zhao. Synthesis and Optoelectronic Studies of Thermally Activated Delayed Fluorescence Materials Based on Benzothiazolyl Ketones [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3876-3887. |
| [7] | Duoduo Xiao, Jiantao Zhang, Peng Zhou, Weibing Liu. Metal-Free α-C(sp3)—H Methylenation of Aryl Ketones to Form γ-Keto Sulfoxides with Dimethyl Sulfoxide [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3900-3906. |
| [8] | Yunpeng Qi, Dengkai Lin, Liang-An Chen. Research Progress on Reductive Acylation with Acyl-Ni as a Key Intermediate to Synthesize Ketones [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3861-3875. |
| [9] | Biao Han, Yaoyao Zhang, Shuhan Chen, Mengge Zhao, Nan Li, Weishuang Li, Lei Zhu. Preparation of Axially Grafted Temperature-Responsive Chiral Salen MnIII and Application in Asymmetric Epoxidation of Olefins in Water [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 244-253. |
| [10] | Cunwei Qian, Rong Han, Zhixing Shen, Qian Li, Xuanrong Chen. N-Iodosuccinimide (NIS) Promoted Synthesis of 3-Substituted Indole Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2496-2503. |
| [11] | Junjiao Wang, Yuyu Lv, Yongwei Shang, Zhenli Cui, Ke-Hu Wang, Danfeng Huang, Yulai Hu. Research Progress of Reactions Participated by α-Hydroxy Ketones [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2300-2321. |
| [12] | Fei Yuan, Yan Zhao, Qingsong Guo, Fudan Yin, Jinrong Lai, Beifang Nian, Ming Zhang, E Tang. Synthesis of 1-[1-(Amino)cyclopropyl]ketones by Tandem Reaction Involving Vinyl Selenium Salt [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1759-1769. |
| [13] | Wenjing Yi, Wei Sun, Xinquan Hu, Chao Liu, Liqun Jin. Recent Advance of Ketones Synthesis from Carboxylic Esters [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1626-1639. |
| [14] | Zhiwei Ma, Xiaopei Chen, Chuanchuan Wang, Jianling Wang, Jingchao Tao, Quanjian Lü. Chiral Squaramide Catalyzed Enantioselective Michael Addition of Cyclic 1,3-Diketones to β,γ-Unsaturated α-Keto Esters [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1520-1526. |
| [15] | JIan Xiao, Zhiying Wu, Ziyi Chen, Pengfei Zhao. Tetraethylenepentamine Functionalized Phenolic Resin as Highly Active Acid-Base Bifunctional Catalyst for Knoevenagel Condensation Reaction [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1179-1187. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||