Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (3): 748-779.DOI: 10.6023/cjoc202311004 Previous Articles Next Articles
Special Issue: 光电催化综述合集
REVIEWS
陈远航, 何劲宇, 张博, 王延钊, 孔令轩, 钱伟烽, 王娜娜, 段闻喜, 欧阳妍妍, 朱翠菊*( ), 徐浩*(
), 徐浩*( )
)
收稿日期:2023-11-03
修回日期:2024-01-23
发布日期:2024-04-02
作者简介:共同第一作者
基金资助:
Yuanhang Chen, Jinyu He, Bo Zhang, Yanzhao Wang, Lingxuan Kong, Weifeng Qian, Na'na Wang, Wenxi Duan, Yanyan Ouyang, Cuiju Zhu( ), Hao Xu(
), Hao Xu( )
)
Received:2023-11-03
Revised:2024-01-23
Published:2024-04-02
Contact:
*E-mail: cuiju.zhu@ccnu.edu.cn; hao.xu@ccnu.edu.cn
About author:These authors contributed equally to this work.
Supported by:Share
Yuanhang Chen, Jinyu He, Bo Zhang, Yanzhao Wang, Lingxuan Kong, Weifeng Qian, Na'na Wang, Wenxi Duan, Yanyan Ouyang, Cuiju Zhu, Hao Xu. Asymmetric Electrochemical Organic Synthesis[J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 748-779.
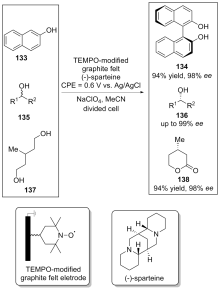
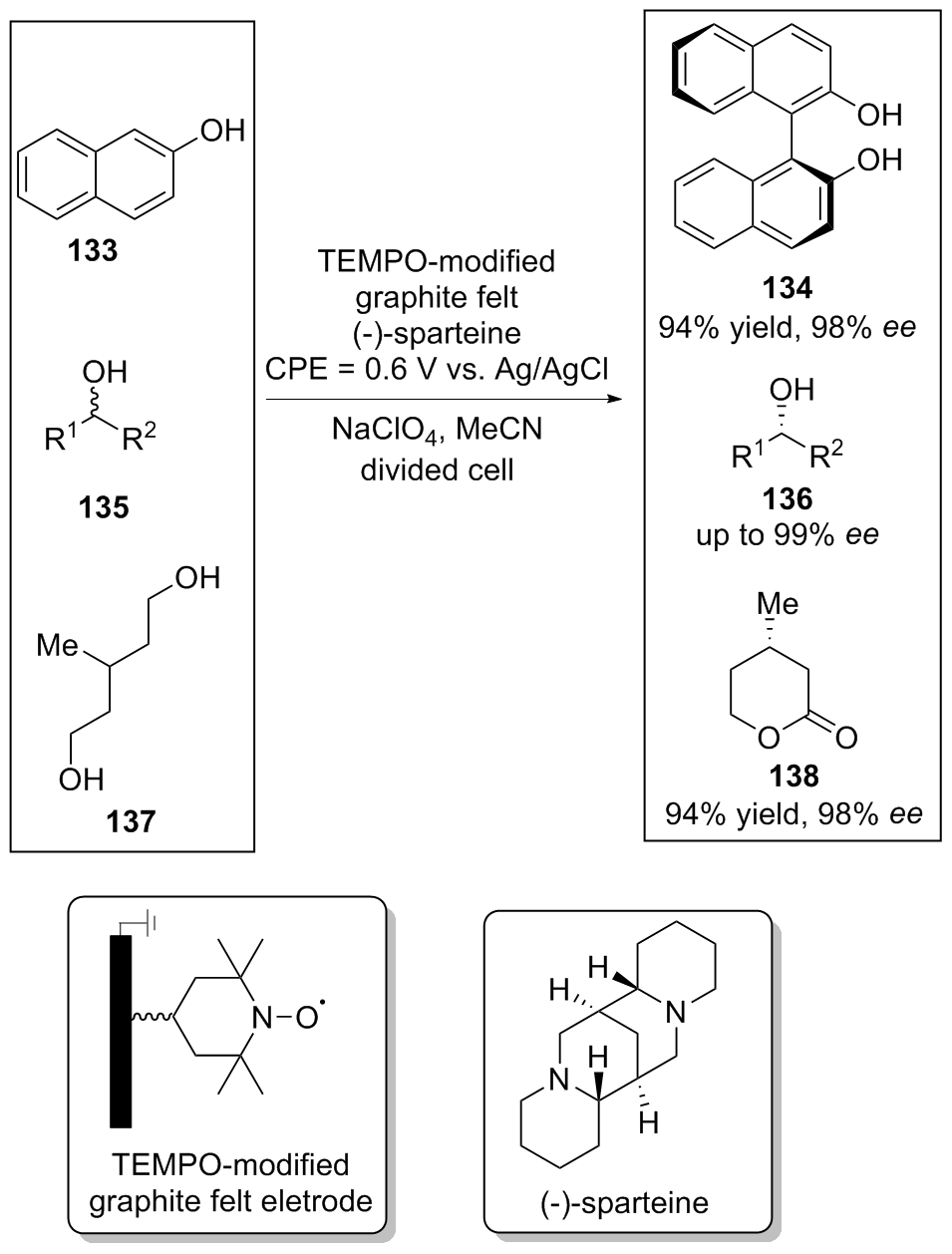
| [1] |
Frontana-Uribe B. A.; Little R. D.; Ibanez J. G.; Palma A.; Vasquez-Medrano R. Green Chem. 2010, 12, 2099.
doi: 10.1039/c0gc00382d |
| [2] |
(a) Wiebe A.; Gieshoff T.; Mohle S.; Rodrigo E.; Zirbes M.; Waldvogel S. R. Angew. Chem., Int. Ed. 2018, 57, 5594.
doi: 10.1002/anie.v57.20 |
|
(b) Guo S.-P.; Liu L.-Y.; Hu K.-F.; Sun Q.; Zha Z.-G.; Yang Y.; Wang Z.-Y. Chin. Chem. Lett. 2021, 32, 1033.
doi: 10.1016/j.cclet.2020.09.041 |
|
|
(c) He W.-B.; Zhao S.-J.; Chen J.-Y.; Jiang J.; Chen X.; Xu X.-H.; He W.-M. Chin. Chem. Lett. 2023, 34, 107640.
doi: 10.1016/j.cclet.2022.06.063 |
|
|
(d) Yang Z.-X.; Lai L.-C.; Chen J.-Z.; Yan H.; Chen F.-E. Chin. Chem. Lett. 2023, 34, 107956.
doi: 10.1016/j.cclet.2022.107956 |
|
| [3] |
(a) Yan M.; Kawamata Y.; Baran P. S. Chem. Rev. 2017, 117, 13230.
doi: 10.1021/acs.chemrev.7b00397 |
|
(b) Li J.-J.; Zhang S.; Xu K. Chin. Chem. Lett. 2021, 32, 2729.
doi: 10.1016/j.cclet.2021.03.027 |
|
| [4] |
Martins G. M.; Shirinfar B.; Hardwick T.; Ahmed N. ChemElectroChem 2019, 6, 1300.
doi: 10.1002/celc.v6.5 |
| [5] |
Wang X.-Y.; Wang Z.-H.; Fang P.; Mei T.-S. Chin. J. Org. Chem. 2020, 40, 3738. (in Chinese)
doi: 10.6023/cjoc202003022 |
|
( 王向阳, 徐学涛, 王振华, 方萍, 梅天胜, 有机化学, 2020, 40, 3738.)
doi: 10.6023/cjoc202003022 |
|
| [6] |
Fu N.-K.; Song L.; Liu J.-J.; Shen Y.-F.; Siu J.-C.; Lin S. J. Am. Chem. Soc. 2019, 141, 14480.
doi: 10.1021/jacs.9b03296 |
| [7] |
Wang Z.-H.; Gao P.-S.; Wang X.; Gao J.-Q.; Xu X.-T.; He Z.; Ma C.; Mei T.-S. J. Am. Chem. Soc. 2021, 143, 15599.
doi: 10.1021/jacs.1c08671 |
| [8] |
(a) Zhang Q.-L.; Chang X.-H.; Peng L.-Z.; Guo C. Angew. Chem. 2019, 131, 7073.
doi: 10.1002/ange.v131.21 |
|
(b) Zhang Q.-L.; Liang K.; Guo C. Angew. Chem. Int. Ed. 2022, 61, e202210632.
doi: 10.1002/anie.v61.38 |
|
|
(c) Zhang Q.-L.; Liang K.; Guo C. CCS Chem. 2021, 3, 338.
doi: 10.31635/ccschem.021.202000720 |
|
|
(d) Liang K.; Zhang Q.-L.; Guo C. Sci. Adv. 2022, 8, eadd7134.
doi: 10.1126/sciadv.add7134 |
|
|
(e) Liang K.; Zhang Q.-L.; Guo C. Nat. Synth. 2023, 2, 1184.
doi: 10.1038/s44160-023-00372-w |
|
| [9] |
Zhou G.; Chen J.-H.; Yao Q.-J.; Huang F.-R.; Wan Z.-K.; Shi B.-F. Angew. Chem. Int. Ed. 2023, 62, e202302964.
doi: 10.1002/anie.v62.21 |
| [10] |
(a) Münchow T.; Dana S.; Xu Y.; Yuan B.-B.; Ackermann L. Science 2023, 379, 1036.
doi: 10.1126/science.adg2866 |
|
(b) Dhawa U.; Tian C.; Wdowik T.; Oliveira J.C.A.; Hao J.-P.; Ackermann L. Angew. Chem. Int. Ed. 2020, 59, 13451.
doi: 10.1002/anie.v59.32 |
|
| [11] |
Franco D.; Riahi A.; Hénin F.; Muzart J.; Duñach E. Eur. J. Org. Chem. 2002, 14, 2257.
|
| [12] |
Magdesieva T. V.; Levitskiy O. A.; Grishin Y. K.; Ambartsumyan A. A.; Kiskin M. A. Organometallics 2014, 33, 4629.
doi: 10.1021/om500070n |
| [13] |
DeLano T. J.; Reisman S. E. ACS Catal. 2019, 9, 6751.
doi: 10.1021/acscatal.9b01785 |
| [14] |
Qiu H.; Shuai B.; Wang Y.-Z.; Liu D.; Chen Y.-G.; Gao P.-S.; Ma H.-X.; Chen S.; Mei T.-S. J. Am. Chem. Soc. 2020, 142, 9872.
doi: 10.1021/jacs.9b13117 |
| [15] |
Wang Y.-Z.; Wang Z.-H.; Eshel I. L.; Sun B.; Liu D.; Gu Y.-C.; Milo A.; Mei T.-S. Nat. Commun. 2023, 14, 2322.
doi: 10.1038/s41467-023-37965-0 |
| [16] |
Sun B.; Wang Z.-H.; Wang Y.-Z.; Gu Y.-C.; Ma C.; Mei T.-S. Sci. Bull. 2023, 68, 2033.
doi: 10.1016/j.scib.2023.07.007 |
| [17] |
Chen B.-L.; Zhu H.-W.; Xiao Y.; Sun Q.-L.; Wang H.; Lu J.-X. Electrochem. Commun. 2014, 42, 55.
doi: 10.1016/j.elecom.2014.02.009 |
| [18] |
Gao S.-G.; Wang C.; Yang J.-F.; Zhang J.-L. Nat. Commun. 2023, 14, 1301.
doi: 10.1038/s41467-023-36704-9 |
| [19] |
Jiao K.-J.; Li Z.-M.; Xu X.-T.; Zhang L.-P.; Li Y.-Q.; Zhang K.; Mei T.-S. Org. Chem. Front. 2018, 5, 2244.
doi: 10.1039/C8QO00507A |
| [20] |
Ding W.-J.; Li M.-F.; Fan J.-K.; Cheng X. Nat. Commun. 2022, 13, 5642.
doi: 10.1038/s41467-022-33452-0 |
| [21] |
Höllrigl V.; Otto K.; Schmid A. Adv. Synth. Catal. 2007, 349, 1337.
doi: 10.1002/adsc.v349:8/9 |
| [22] |
Torii S.; Liu P.; Bhuvaneswari N.; Amatore C.; Jutand A. J. Org. Chem. 1996, 61, 3055.
doi: 10.1021/jo952137r |
| [23] |
Tanaka H.; Kuroboshi M.; Takeda H.; Kanda H.; Torii S. J. Electroanal. Chem. 2001, 507, 75.
doi: 10.1016/S0022-0728(01)00387-4 |
| [24] |
Huang X.-Q.; Zhang Q.; Lin J.-H.; Harms K. ; Meggers E. Nat. Catal. 2019, 2, 34.
doi: 10.1038/s41929-018-0198-y |
| [25] |
Xiong P.; Marcel H.; Ivlev S. I.; Meggers E. J. Am. Chem. Soc. 2022, 144, 6964.
doi: 10.1021/jacs.2c01686 pmid: 35385651 |
| [26] |
Huang Y.-Q.; Wu Z.-J.; Zhu L.; Gu Q.; Lu X.-J.; You S.-L.; Mei T.-S. CCS Chem. 2022, 4, 3181.
doi: 10.31635/ccschem.021.202101376 |
| [27] |
Minato D.; Arimoto H.; Nagasue Y.; Demizu Y.; Onomura O. Tetrahedron 2008, 64, 6675.
doi: 10.1016/j.tet.2008.05.015 |
| [28] |
Gao P.-S.; Weng X.-J.; Wang Z.-H.; Zheng C.; Sun B.; Chen Z.-H.; You S.-L.; Mei T.-S. Angew. Chem. Int. Ed. 2020, 59, 15254.
doi: 10.1002/anie.v59.35 |
| [29] |
(a) Cai C.-Y.; Lai X.-L.; Wang Y.; Hu H.-H.; Song J.-S.; Yang Y.; Wang C.; Xu H.-C. Nat. Catal. 2022, 5, 943.
doi: 10.1038/s41929-022-00855-7 |
|
(b) Fan W.-Z.; Zhao X.-Y.; Deng Y.-S.; Chen P.-H.; Wang F.; Liu G.-S. J. Am. Chem. Soc. 2022, 47, 21674.
|
|
| [30] |
Lai X.-L.; Chen M.; Wang Y.-Q.; Song J.-S.; Xu H.-C. J. Am. Chem. Soc. 2022, 144, 20201.
doi: 10.1021/jacs.2c09050 |
| [31] |
Lai X.-L.; Xu H.-C. J. Am. Chem. Soc. 2023, 145, 18753.
doi: 10.1021/jacs.3c07146 |
| [32] |
Kise N.; Iwasaki K.; Tokieda N.; Ueda N. Org. Lett. 2001, 3, 3241.
pmid: 11594804 |
| [33] |
Palombi L.; Feroci M.; Orsinia M.; Inesi A. Tetrahedron 2002, 13, 2311.
|
| [34] |
(a) Feroci M.; Inesi A.; Orsini M.; Palombi L. Org. Lett. 2002, 4, 2617.
pmid: 14725464 |
|
(b) Feroci M.; Orsini M.; Palombi L.; Sotgiu G.; Colapietro M.; Inesi A. J. Org. Chem. 2004, 69, 487.
pmid: 14725464 |
|
| [35] |
Zhang K.; Wang H.; Zhao S.-F.; Niu D.-F.; Lu J.-X. J. Electroanal. Chem. 2009, 630, 35.
doi: 10.1016/j.jelechem.2009.02.013 |
| [36] |
Lütz S.; Steckhan E.; Liese A. Electrochem. Commun. 2004, 6, 583.
doi: 10.1016/j.elecom.2004.04.009 |
| [37] |
Hollmann F.; Hofstetter K.; Habicher T.; Hauer B.; Schmid A. J. Am. Chem. Soc. 2005, 127, 6540.
doi: 10.1021/ja050997b pmid: 15869268 |
| [38] |
Feroci M. Adv. Synth. Catal. 2007, 349, 2177.
doi: 10.1002/adsc.v349:13 |
| [39] |
Yang H.-P.; Lv T.; Sun W.-W.; Du Y.-F.; Wang H.; Lu J.-X. RSC Adv. 2014, 4, 30584.
doi: 10.1039/C4RA03368J |
| [40] |
Yue Y.-N.; Wu D.; Zeng S.; Yang M.-P.; Wang H.; Lu J.-X. New J. Chem. 2017, 41, 7853.
doi: 10.1039/C7NJ00844A |
| [41] |
Chen H.; Cai R.; Patel J.; Dong F.-Y.; Chen H.; Minteer S.D. J. Am. Chem. Soc. 2019, 141, 4963.
doi: 10.1021/jacs.9b00147 pmid: 30835461 |
| [42] |
Wang Z.-L.; Zhao Y.-J.; Xiong R.; Yang L.-R.; Wang H.; Lu J.-X. ChemistrySelect 2021, 6, 876.
doi: 10.1002/slct.v6.4 |
| [43] |
(a) Osa T.; Kashiwagi Y.; Yanagisawa Y.; Bobbitt J. M. J. Chem. Soc. Chem. Commun. 1994, 2535.
|
|
(b) Kashiwagi Y.; Yanagisawa Y.; Kurashima F.; Bobbitt J. M. Chem. Commun. 1996, 2745.
|
|
|
(c) Yanagisawa Y.; Kashiwagi Y.; Kurashima F.; Bobbitt J. M. Chem. Lett. 1996, 25, 1043.
doi: 10.1246/cl.1996.1043 |
|
| [44] |
Maekawa H.; Itoh K.; Goda S.; Nishiguchi I. Chirality 2003, 15, 95.
doi: 10.1002/chir.v15:1 |
| [45] |
Sierecki E.; Turcaud S.; Royer J.; Martens T. Synthesis 2006, 19, 3199.
|
| [46] |
Page P.C.B.; Marken F.; Williamson C.; Chan Y.; Buckley B.R.; Bethell D. Adv. Synth. Catal. 2008, 350, 1149.
doi: 10.1002/adsc.v350:7/8 |
| [47] |
Shiigi H.; Mori H.; Tanaka T.; Demizu Y.; Onomura O. Tetrahedron Lett. 2008, 49, 5247.
|
| [48] |
Lee D.-S. Tetrahedron 2009, 20, 2014.
|
| [49] |
D'Oca, M. G. M.; Pilli, R. A.; Vencato, I. Tetrahedron Lett. 2000, 41, 9709.
doi: 10.1016/S0040-4039(00)01749-4 |
| [50] |
Jensen K. L.; Franke P. T.; Nielsen L. T.; Daasbjerg K.; Jørgensen K. A. Angew. Chem. Int. Ed. 2010, 49, 129.
doi: 10.1002/anie.200904754 pmid: 19946923 |
| [51] |
Bui N.-N.; Ho X.-H.; Mho S.; Jang H.-Y. Eur. J. Org. Chem. 2009, 5309.
|
| [52] |
Ho X.-H.; Mho S.-I.; Kang H.; Jang H.-Y. Eur. J. Org. Chem. 2010, 4436.
|
| [53] |
Fu N.-K.; Li L.-J.; Yang Q.; Luo S.-Z. Org. Lett. 2017, 19, 2122.
doi: 10.1021/acs.orglett.7b00746 |
| [54] |
Gao W.-C.; Xiong Z.-Y.; Pirhaghani S.; Wirth T. Synthesis 2019, 51, 276.
doi: 10.1055/s-0037-1610373 |
| [55] |
Li L.-J.; Li Y.; Fu N.-K.; Zhang L.; Luo S.-Z. Angew. Chem. Int. Ed. 2020, 59, 14347.
doi: 10.1002/anie.v59.34 |
| [56] |
Lu F.-Y.; Chen Y.-J.; Chen Y.; Ding X.; Guan Z.; He Y.-H. Chem. Commun. 2020, 56, 623.
doi: 10.1039/C9CC09178E |
| [57] |
Chang X.-H.; Zhang J.-Y.; Zhang Q.-L.; Guo C. Angew. Chem. Int. Ed. 2020, 59, 18500.
doi: 10.1002/anie.v59.42 |
| [1] | Tao Zeng, Shusheng Zhang, Jianguo Fu, Chenguo Feng. Asymmetric Synthesis of Hamaudol and sec-O-Glucosylhamaudol in Saposhnikovia divaricata (Turcz.) Schischk [J]. Chinese Journal of Organic Chemistry, 2024, 44(9): 2862-2868. |
| [2] | Ming Zhao, Rui Yan, Hu Chen. N-Heterocyclic Carbene Catalyzed the Umpolung of Aldehyde Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(7): 2204-2215. |
| [3] | Lingyi Lu, Xiaodong Qiu. Recent Progress in Radical Involved Alkene Dialkylation [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1701-1718. |
| [4] | Leilei Liang, Jiacan Yao, Fan Ding, Chang Xu, Dandan Liu. Research Progress on the Synthetic Methods of Pancratistatin [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1793-1810. |
| [5] | Chen-Long Li, Zhi-Xiang Yu. Progress in Transition-Metal-Catalyzed Carbonylative Cycloadditions Using Carbon Monoxide [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1045-1068. |
| [6] | Kaijie Guo, Xinshu Fu, Jing Li, Yan Chen, Meili Hu, Xihua Du, Yuyang Xie, Yan He. Recent Advances in Transition-Metal-Catalyzed C—S Bond Activation and Transformations [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1124-1150. |
| [7] | Xinyue Fang, Yawen Huang, Xinwei Hu, Zhixiong Ruan. Recent Progress in Electrochemical Modification of Amino Acids and Peptides [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 903-926. |
| [8] | Mengfan Li, Xu Cheng. Chemoselective Electro-oxidation of Allyl Arene to Ester [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 1005-1012. |
| [9] | Zhang-Jian Li, Zhen-Hua Wang, Jian-Feng Guo, Ping Fang, Cong Ma, Run-Hua Liu, Tian-Sheng Mei. Electrochemistry-Enabled 2,2,6,6-Tetramethylpiperidoxyl (TEMPO)-Mediated Oxidative Dehydrogenation Povarov/Tandem Reactions of Glycine Derivatives [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 940-950. |
| [10] | Chun Gao, Xin Liu, Minghui Wang, Shuxian Liu, Tingting Zhu, Yikang Zhang, Erjun Hao, Qiliang Yang. Advances in Asymmetric Electrochemical Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 673-727. |
| [11] | Aman Hasil, Rui Chang, Juntao Ye. Recent Advances on C—H Functionalization via Oxidative Electrophotocatalysis [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 728-747. |
| [12] | Shuai Lv, Gangguo Zhu, Jinzhong Yao, Hongwei Zhou. Research Progress in Preparation of Carboxylic Acids by Electrochemical Mediated Oxidative Carboxylation and Reductive Carboxylation of Carbon Dioxide [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 780-808. |
| [13] | Jian Huang, Wenzhen Zhang. Advances in Electrochemical Cathodic Reductive Reactions Involving Carbon-Nitrogen Bonds [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 825-839. |
| [14] | Lan Zhou, Hong He, De-Qiao Yang, Zhong-Wei Hou, Lei Wang. Electrochemical Trifluoromethylation/Spirocyclization of N-Benzylacrylamides to Construct Trifluoromethylated 2-Azaspiro[4.5]decanes [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 981-988. |
| [15] | Jiwei Wu, Jun He, Jingjing Wang, Lixia Li, Caiyu Xu, Jie Zhou, Zirong Li, Huajian Xu. Electrochemical Oxidation Decarboxylative Cyclization of α-Keto Acid with o-Aminobenzylamine [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 972-980. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||