Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (9): 2841-2846.DOI: 10.6023/cjoc202405006 Previous Articles Next Articles
ARTICLES
收稿日期:2024-05-07
修回日期:2024-05-17
发布日期:2024-05-23
通讯作者:
陈丰坤
基金资助:
Yufei Xia, Li Jiang, Qiao Yang, Xiu Yu, Fengkun Chen( )
)
Received:2024-05-07
Revised:2024-05-17
Published:2024-05-23
Contact:
Fengkun Chen
Supported by:Share
Yufei Xia, Li Jiang, Qiao Yang, Xiu Yu, Fengkun Chen. Triple Aza[6]helicenes with Circularly Polarized Luminescence: N-Alkylation as a Tool to Tune the Chiroptical Properties[J]. Chinese Journal of Organic Chemistry, 2024, 44(9): 2841-2846.
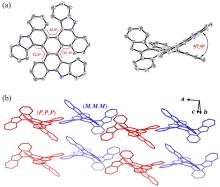
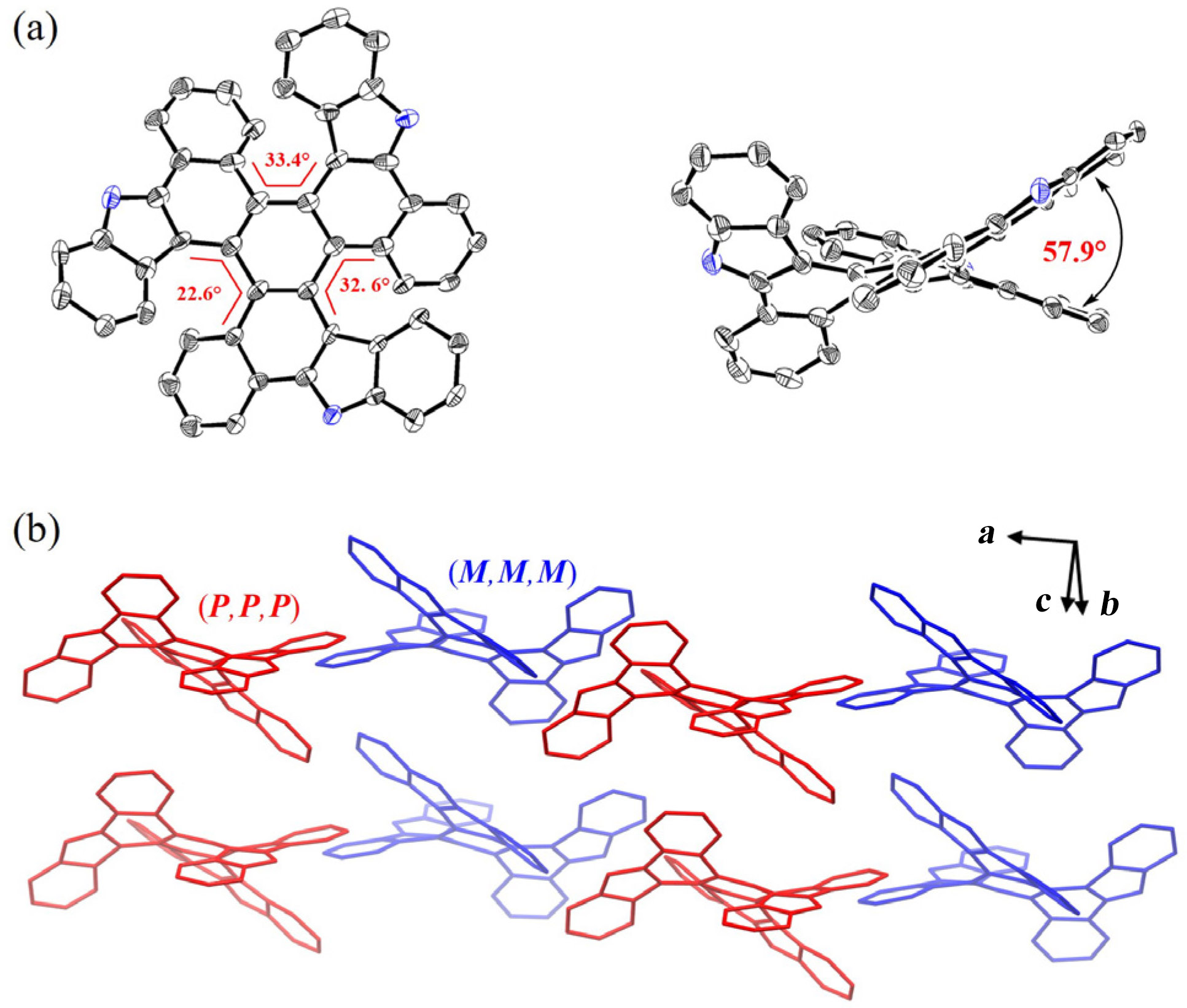
| Compound | λabsb/nm | λemb/nm | | ΦPLd/% | τse/ns | kr f/(107 s-1) | knr/(108 s-1) |
|---|---|---|---|---|---|---|---|
| TO6Ha | 365 | 490/510 | — | 5.0 | 5.80 | 0.86 | 1.64 |
| 2 | 388 | 506/530 | 2.46 | 5.5 | 4.27 | 1.29 | 2.21 |
| 3 | 393 | 508/532 | 2.44 | 5.8 | 4.41 | 1.32 | 2.14 |
| Compound | λabsb/nm | λemb/nm | | ΦPLd/% | τse/ns | kr f/(107 s-1) | knr/(108 s-1) |
|---|---|---|---|---|---|---|---|
| TO6Ha | 365 | 490/510 | — | 5.0 | 5.80 | 0.86 | 1.64 |
| 2 | 388 | 506/530 | 2.46 | 5.5 | 4.27 | 1.29 | 2.21 |
| 3 | 393 | 508/532 | 2.44 | 5.8 | 4.41 | 1.32 | 2.14 |
| [1] |
Petrukhina, M. A.; Scott, L. T. Fragments of Fullerenes and Carbon Nanotubes: Designed Synthesis, Unusual Reactions, and Coordination Chemistry, Wiley, Hoboken, New Jersey, 2011.
|
| [2] |
(a) Pun, S. H.; Miao, Q. Acc. Chem. Res. 2018, 51, 1630.
|
|
(b) Chao, L.; Stepek, I. A.; Yamada, K. E.; Ito, H.; Itami, K. Angew. Chem., Int. Ed. 2021, 60, 23508.
|
|
|
(c) Majewski, M. A.; Stępień, M. Angew. Chem., nt. Ed. 2019, 58, 86.
|
|
| [3] |
(a) Shen, Y.; Chen, C.-F. Chem. Rev. 2012, 112, 1463.
doi: 10.1021/cr200087r pmid: 23151799 |
|
(b) Gingras, M. Chem. Soc. Rev. 2013, 42, 968.
doi: 10.1039/c2cs35154d pmid: 23151799 |
|
|
(c) Crassous, J.; Stará, I. G.; Starý, I. Helicenes: Synthesis, Properties and Applications, Wiley-VCH, Weinheim, 2022.
pmid: 23151799 |
|
| [4] |
(a) Fang, L.; Lin, W.; Shen, Y.; Chen, C.-F. Chin. J. Org. Chem. 2018, 38, 541 (in Chinese).
|
|
(房蕾, 林伟彬, 沈赟, 陈传峰, 有机化学, 2018, 38, 541.)
doi: 10.6023/cjoc201710028 |
|
|
(b) Narcis, M. J.; Takenaka, N. Eur. J. Org. Chem. 2014, 2014, 21.
|
|
| [5] |
(a) Zhao, W.-L.; Li, M.; Lu, H.-Y.; Chen, C.-F. Chem. Commun. 2019, 55, 13793.
|
|
(b) Mori, T. Chem. Rev. 2021, 121, 2373.
|
|
| [6] |
(a) Shen, C.; Gan, F.; Zhang, G.; Ding, Y.; Wang, J.; Wang, R.; Crassous, J.; Qiu, H. Mater. Chem. Front. 2020, 4, 837.
|
|
(b) Kubo, H.; Hirose, T.; Nakashima, T.; Kawai, T.; Hasegawa, J.-Y.; Matsuda, K. J. Phys. Chem. Lett. 2021, 12, 686.
|
|
|
(c) Shen, Y.-J.; Yao, N.-T.; Diao, L.-N.; Yang, Y.; Chen, X.-L.; Gong, H.-Y. Angew. Chem., nt. Ed. 2023, 62, e202300840.
|
|
|
(d) Huo, G.-F.; Xu, W.-T.; Han, Y.; Zhu, J.; Hou, X.; Fan, W.; Ni, Y.; Wu, S.; Yang, H.-B.; Wu, J. Angew. Chem., nt. Ed. 2024, 63, e202403149.
|
|
| [7] |
(a) Kiran, V.; Mathew, S. P.; Cohen, S. R.; Hernández Delgado, I.; Lacour, J.; Naaman, R. Adv. Mater. 2016, 28, 1957.
|
|
(b) Pan, T.-R.; Guo, A.-M.; Sun, Q.-F. Phys. Rev. B 2016, 94, 235448.
|
|
|
(c) Kettner, M.; Maslyuk, V. V.; Nürenberg, D.; Seibel, J.; Gutierrez, R.; Cuniberti, G.; Ernst, K.-H.; Zacharias, H. J. Phys. Chem. Lett. 2018, 9, 2025.
|
|
|
(d) Rodríguez, R.; Naranjo, C.; Kumar, A.; Matozzo, P.; Das, T. K.; Zhu, Q.; Vanthuyne, N.; Gómez, R.; Naaman, R.; Sánchez, L.; Crassous, J. J. Am. Chem. Soc. 2022, 144, 7709.
|
|
| [8] |
(a) Li, C.; Yang, Y.; Miao, Q. Chem.-Asian J. 2018, 13, 884.
|
|
(b) Wu, Y.-F.; Zhang, L.; Zhang, Q.; Xie, S.-Y.; Zheng, L.-S. Org. Chem. Front. 2022, 9, 4726.
|
|
| [9] |
(a) Zhu, Y.; Xia, Z.; Cai, Z.; Yuan, Z.; Jiang, N.; Li, T.; Wang, Y.; Guo, X.; Li, Z.; Ma, S.; Zhong, D.; Li, Y.; Wang, J. J. Am. Chem. Soc. 2018, 140, 4222.
pmid: 37428944 |
|
(b) Chen, F.; Gu, W.; Saeki, A.; Melle-Franco, M.; Mateo-Alonso, A. Org. Lett. 2020, 22, 3706.
pmid: 37428944 |
|
|
(c) Artigas, A.; Rigoulet, F.; Giorgi, M.; Hagebaum-Reignier, D.; Carissan, Y.; Coquerel, Y. J. Am. Chem. Soc. 2023, 145, 15084.
doi: 10.1021/jacs.3c05415 pmid: 37428944 |
|
| [10] |
Hosokawa, T.; Takahashi, Y.; Matsushima, T.; Watanabe, S.; Kikkawa, S.; Azumaya, I.; Tsurusaki, A.; Kamikawa, K. J. Am. Chem. Soc. 2017, 139, 18512.
doi: 10.1021/jacs.7b07113 pmid: 28875702 |
| [11] |
(a) Tanaka, H.; Ikenosako, M.; Kato, Y.; Fujiki, M.; Inoue, Y.; Mori, T. Commun. Chem. 2018, 1, 38.
|
|
(b) Niu, W.; Fu, Y.; Qiu, Z.-L.; Schürmann, C. J.; Obermann, S.; Liu, F.; Popov, A. A.; Komber, H.; Ma, J.; Feng, X. J. Am. Chem. Soc. 2023, 145, 26824.
|
|
| [12] |
Yang, W.-W.; Shen, J.-J. Chem.-Eur. J. 2022, 28, e202202069.
|
| [13] |
(a) Guo, X.; Yuan, Z.; Zhu, Y.; Li, Z.; Huang, R.; Xia, Z.; Zhang, W.; Li, Y.; Wang, J. Angew. Chem., nt. Ed. 2019, 58, 16966.
|
|
(b) Li, J.-K.; Chen, X.-Y.; Guo, Y.-L.; Wang, X.-C.; Sue, A. C. H.; Cao, X.-Y.; Wang, X.-Y. J. Am. Chem. Soc. 2021, 143, 17958.
|
|
|
(c) Zhang, L.; Chen, S.; Jiang, J.; Dong, X.; Cai, Y.; Zhang, H.-J.; Lin, J.; Jiang, Y.-B. Org. Lett. 2022, 24, 3179.
|
|
|
(d) Tan, D.; Dong, J.; Ma, T.; Feng, Q.; Wang, S.; Yang, D.-T. Angew. Chem., Int. Ed. 2023, 62, e202304711.
|
|
|
(e) Sun, Z.; Xu, W.; Qiu, S.; Ma, Z.; Li, C.; Zhang, S.; Wang, H. Chem. Sci. 2024, 15, 1077.
|
|
| [14] |
(a) Feng, X.; Wu, J.; Enkelmann, V.; Müllen, K. Org. Lett. 2006, 8, 1145.
|
|
(b) Feng, X.; Wu, J.; Ai, M.; Pisula, W.; Zhi, L.; Rabe, J. P.; Müllen, K. Angew. Chem., nt. Ed. 2007, 46, 3033.
|
|
|
(c) Liu, Y.; Marszalek, T.; Müllen, K.; Pisula, W.; Feng, X. Chem.- Asian J. 2016, 11, 2107.
|
|
| [15] |
Liu, J.; Jiang, L.; Chang, H.; Liu, H.; Cao, X.-Y.; Zou, Y.; Hu, Y. Chem. Commun. 2022, 58, 13087.
|
| [16] |
(a) Chen, F.; Hong, Y. S.; Shimizu, S.; Kim, D.; Tanaka, T.; Osuka, A. Angew. Chem., nt. Ed. 2015, 54, 10639.
|
|
(b) Chen, F.; Hong, Y. S.; Kim, D.; Tanaka, T.; Osuka, A. ChemPlusChem 2017, 82, 1048.
|
|
|
(c) Matsuo, Y.; Chen, F.; Kise, K.; Tanaka, T.; Osuka, A. Chem. Sci. 2019, 10, 11006.
|
|
| [17] |
Zhou, F.; Huang, Z.; Huang, Z.; Cheng, R.; Yang, Y.; You, J. Org. Lett. 2021, 23, 4559.
|
| [1] | Jianling Hu, Chao Zhang, Wenda Zhu, Yepu He, Shuling Peng, Zhenqiang Chen, Mingyue Li, Zhiju Liu, Heru Chen. Design, Synthesis, and Preliminary Anti-tumor Activity Studies of Novel 1,2-Disubstituted Hydrazines [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1870-1883. |
| [2] | Junjun Liu, Taotao Lu, Ping Ma, Qingyang Zhao, Fuk Yee Kwong. Palladium-Catalyzed C(sp3)—Si Bonds Transformation for Construct-ing Trifluoropropyl (Hetero)arenes through C(sp3)—C(sp2) Cross-Coupling Reactions [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1319-1326. |
| [3] | Xiao Song, Jing Qing, Jun Li, Xuelei Jia, Fusong Wu, Junrong Huang, Jian Jin, Hengzhi You. Copper-Catalyzed Asymmetric Allyl Alkylation Using Grignard Reagents under Continuous Flow [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3174-3179. |
| [4] | Chun-Xia Cheng, Lu-Ping Wu, Feng Sha, Xin-Yan Wu. Enantioselective Vinylogous Allylic Alkylation of Coumarins with Morita-Baylis-Hillman Carbonates Catalyzed by Chiral Phosphine-Amide [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3188-3195. |
| [5] | Boyu Yan, Jieliang Wu, Jinfei Deng, Dan Chen, Xiushen Ye, Qiuli Yao. Recent Progress in Light-Driven Direct Dehydroxylation and Derivation of Alcohols [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3055-3066. |
| [6] | Yingke Feng, He Wang, Mengxing Cui, Ran Sun, Xin Wang, Yang Chen, Lei Li. Visible-Light-Induced Difluoroalkylated Cyclization of Novel Functionalized Aromatic Isocyanides [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2913-2925. |
| [7] | Fen Huang, Weiwei Luo, Jun Zhou. Research Progress of Polychloroalkylation Based on C—H Bond Cleavage [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2368-2390. |
| [8] | Li Sun, Guoxin Song, Jiale Han, Jiyu Li, Yue Zhao, Luhua Yang, Feng Zhang, Kun Zhao, Biming Mao. Electrochemical Allylic Alkylation of Morita-Baylis-Hillman Adducts and N-Hydroxyphthalimide Esters towards C(sp3)—C(sp3) Bond Formation [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1574-1583. |
| [9] | Jinxiao Zhao, Tonghui Wei, Sen Ke, Yi Li. Visible Light-Catalyzed Synthesis of Difluoroalkylated Polycyclic Indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1102-1114. |
| [10] | Peng Zhou, Weiming Zhu, Jiantao Zhang, Duoduo Xiao, Xiangfeng Guo, Weibing Liu. Cobalt-Catalyzed Oxyalkylation Reaction of Styrenes: Rapid Access to α-Alkyl Substituted Acetophenone Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3939-3944. |
| [11] | Tiantian Liu, Xinhong Duan. Recent Progress in the Construction of Chiral 3-Substituted Indoles by Asymmetric Friedel-Crafts Reactions [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3695-3712. |
| [12] | Zhi Tu, Jinsheng Yu, Jian Zhou. Synthesis of (Bromodifluoromethyl)trimethylsilane and Its Applications in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3491-3507. |
| [13] | Tianyu Chen, Feng Han, Shuangyan Li, Jianping Liu, Jianhui Chen, Qing Xu. Transition Metal-Free Selective Aerobic C-Alkylation of Methyl N-Heteroarenes with Alcohols [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2914-2924. |
| [14] | Qi Sun, Zeying Sun, Ze Yu, Guangwei Wang. Nickel-Catalyzed Stereoselective Aryl-Difluoroalkylation of Alkynes [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2515-2520. |
| [15] | Xinling Li, Weidong Meng, Xiuhua Xu, Yan'gen Huang. Visible Light Induced Arylfluoroalkylation of Activated Alkenes [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1820-1830. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||