Chinese Journal of Organic Chemistry ›› 2019, Vol. 39 ›› Issue (11): 3207-3214.DOI: 10.6023/cjoc201904069 Previous Articles Next Articles
李恒超, 赵玲, 刘燕, 张霞, 李王兵, 敬林海, 黄锦*( ), 汪伟*(
), 汪伟*( )
)
收稿日期:2019-04-27
发布日期:2019-07-03
通讯作者:
黄锦,汪伟
E-mail:huahuanhuangjin@163.com;wangwei1987@cwnu.edu.cn
基金资助:
Li Hengchao, Zhao Ling, Liu Yan, Zhang Xia, Li Wangbing, Jing Linhai, Huang Jin*( ), Wang Wei*(
), Wang Wei*( )
)
Received:2019-04-27
Published:2019-07-03
Contact:
Huang Jin,Wang Wei
E-mail:huahuanhuangjin@163.com;wangwei1987@cwnu.edu.cn
Supported by:Share
Li Hengchao, Zhao Ling, Liu Yan, Zhang Xia, Li Wangbing, Jing Linhai, Huang Jin, Wang Wei. An Efficient Palladium Nanoparticles Catalytic System for Suzuki Coupling Reactions[J]. Chinese Journal of Organic Chemistry, 2019, 39(11): 3207-3214.
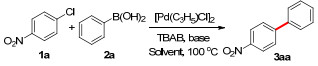 | |||||
| Entry | Base | Solvent | TBAB/mmol | Time/h | Yieldb/% |
| 1 | Et3N | DMA | 0.375 | 8 | 0 |
| 2 | NaOAc | DMA | 0.375 | 8 | 0 |
| 3 | NaHCO3 | DMA | 0.375 | 8 | 10 |
| 4 | Na2CO3 | DMA | 0.375 | 8 | 17 |
| 5 | K2CO3 | DMA | 0.375 | 8 | 100 |
| 6 | K3PO4 | DMA | 0.375 | 8 | 88 |
| 7 | NaOH | DMA | 0.375 | 8 | 19 |
| 8 | K2CO3 | DMA | 0.375 | 2 | 96 |
| 9 | K2CO3 | DMF | 0.375 | 2 | 100 |
| 10 | K2CO3 | 1, 4-Dioxane | 0.375 | 2 | 58 |
| 11 | K2CO3 | n-Butanol | 0.375 | 2 | 26 |
| 12 | K2CO3 | Toluene | 0.375 | 2 | 63 |
| 13 | K2CO3 | DMF | 0.125 | 1 | 94 |
| 14 | K2CO3 | DMF | 0.25 | 1 | 85 |
| 15 | K2CO3 | DMF | 0.375 | 1 | 79 |
| 16 | K2CO3 | DMF | 0.5 | 1 | 84 |
 | |||||
| Entry | Base | Solvent | TBAB/mmol | Time/h | Yieldb/% |
| 1 | Et3N | DMA | 0.375 | 8 | 0 |
| 2 | NaOAc | DMA | 0.375 | 8 | 0 |
| 3 | NaHCO3 | DMA | 0.375 | 8 | 10 |
| 4 | Na2CO3 | DMA | 0.375 | 8 | 17 |
| 5 | K2CO3 | DMA | 0.375 | 8 | 100 |
| 6 | K3PO4 | DMA | 0.375 | 8 | 88 |
| 7 | NaOH | DMA | 0.375 | 8 | 19 |
| 8 | K2CO3 | DMA | 0.375 | 2 | 96 |
| 9 | K2CO3 | DMF | 0.375 | 2 | 100 |
| 10 | K2CO3 | 1, 4-Dioxane | 0.375 | 2 | 58 |
| 11 | K2CO3 | n-Butanol | 0.375 | 2 | 26 |
| 12 | K2CO3 | Toluene | 0.375 | 2 | 63 |
| 13 | K2CO3 | DMF | 0.125 | 1 | 94 |
| 14 | K2CO3 | DMF | 0.25 | 1 | 85 |
| 15 | K2CO3 | DMF | 0.375 | 1 | 79 |
| 16 | K2CO3 | DMF | 0.5 | 1 | 84 |
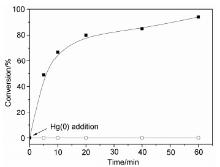
| [1] |
Miyaura N.; Suzuki A. Chem. Rev. 1995, 95 2457.
doi: 10.1021/cr00039a007 |
| [2] | Suzuki A. Chem. Commun. 2005 4759. |
| [3] |
Suzuki A. Angew. Chem., Int. Ed. 2011, 50 6722.
doi: 10.1002/anie.201101379 |
| [4] |
Capdeville R.; Buchdunger E.; Zimmermann J.; Matter A. Nat. Rev. Drug Discovery 2002, 1 493.
doi: 10.1038/nrd839 |
| [5] |
Corbet J. P.; Mignani G. Chem. Rev. 2006, 106 2651.
doi: 10.1021/cr0505268 |
| [6] |
Magano J.; Dunetz J. R. Chem. Rev. 2011, 111 2177.
doi: 10.1021/cr100346g |
| [7] |
Fihri A.; Bouhrara M.; Nekoueishahraki B.; Basset J.-M.; Polshettiwar V. Chem. Soc. Rev. 2011, 40 5181.
doi: 10.1039/c1cs15079k |
| [8] |
(a) Jana, R.; Pathak, T. P.; Sigman, M. S. Chem. Rev. 2011, 111, 1417.
doi: 10.1021/cr100327p |
|
(b) Stockton, K. P.; Merritt, C. J.; Sumby, C. J.; Greatrex, B. W. Eur. J. Org. Chem. 2015, 6999.
doi: 10.1021/cr100327p |
|
|
(c) Li, J.-X.; Yang, S.-R.; Wu, W.-Q.; Jiang, H.-F. Eur. J. Org. Chem. 2018, 1284.
doi: 10.1021/cr100327p |
|
| [9] | Albisson D. A.; Bedford R. B.; Lawrence S. E.; Scully P. N. Chem. Commun. 1998 2095. |
| [10] |
Zapf A.; Ehrentraut A.; Beller M. Angew. Chem., Int. Ed. 2000, 39 4153.
doi: 10.1002/1521-3773(20001117)39:22<4153::AID-ANIE4153>3.0.CO;2-T |
| [11] |
Yuan D.; Huynh H. V. Organometallics 2010, 29 6020.
doi: 10.1021/om1008023 |
| [12] | Bianchini C.; Lee H. M.; Meli A.; Oberhauser W.; Vizza F.; Brüggeller P.; Rainer H. B.; Langes C. Chem. Commun. 2000 777. |
| [13] |
Nair P.; Anderson G. K.; Rath N. P. Organometallics 2003, 22 1494.
doi: 10.1021/om0209069 |
| [14] |
Imamoto T.; Yashio K.; Crépy K. V. L.; Katagiri K.; Takahashi H.; Kouchi M.; Gridnev I. D. Organometallics 2006, 25 908.
doi: 10.1021/om050759p |
| [15] |
Field L. D.; Messerle B. A.; Smernik R. J.; Hambley T. W.; Turner P. Inorg. Chem. 1997, 36 2884.
doi: 10.1021/ic970030b |
| [16] |
(a) Laurenti, D.; Feuerstein, M.; Pèpe, G.; Doucet, H.; Santelli, M. J. Org. Chem. 2001, 66, 1633.
doi: 10.1021/jo001146j |
|
(b) Feuerstein, M.; Laurenti, D.; Bougeant, C.; Doucet, H.; Santelli, M. Chem. Commun. 2001, 325.
doi: 10.1021/jo001146j |
|
|
(c) Feuerstein, M.; Doucet, H.; Santelli, M. Synlett 2001, 9, 1458.
doi: 10.1021/jo001146j |
|
|
(d) Feuerstein, M.; Doucet, H.; Santelli, M. Tetrahedron Lett. 2001, 42, 6667.
doi: 10.1021/jo001146j |
|
|
(e) Feuerstein, M.; Doucet, H.; Santelli, M. J. Organomet. Chem. 2003, 687, 327.
doi: 10.1021/jo001146j |
|
| [17] |
Hierso J.-C.; Fihri A.; Amardeil R.; Meunier P.; Doucet H.; Santelli M.; Donnadieu B. Organometallics 2003, 22 4490.
doi: 10.1021/om0302948 |
| [18] |
Zaborova E.; Deschamp J.; Guieu S.; Bleriot Y.; Poli G.; Menand M.; Madec D.; Prestat G.; Sollogoub M. Chem. Commun. 2011, 47 9206.
doi: 10.1039/c1cc12241j |
| [19] |
(a) Wang, K.; Yi, T.; Yu, X.-J.; Zheng, X.-L.; Fu, H.-Y.; Chen, H.; Li, R.-X. Appl. Organomet. Chem. 2012, 26, 342.
doi: 10.1002/aoc.2869 |
|
(b) Wang, K.; Fu, Q.; Zhou, R.; Zheng, X.-L.; Fu, H.-Y.; Chen, H.; Li, R.-X. Appl. Organomet. Chem. 2013, 27, 232.
doi: 10.1002/aoc.2869 |
|
|
(c) Wang, K.; Wang, W.; Luo, H.; Zheng, X.-L.; Fu, H.-Y.; Chen, H.; Li, R.-X. Catal. Lett. 2013, 143, 1214.
doi: 10.1002/aoc.2869 |
|
|
(d) Guo, F.-C.; Zhou, R.; Jiang, Z.-J.; Wang, W.; Zheng, X.-L.; Fu, H.-Y.; Chen, H.; Li, R.-X. Catal. Commun. 2015, 66, 87.
doi: 10.1002/aoc.2869 |
|
| [20] |
(a) Bernhardt, E.; Willner, H.; Jonas, V.; Thiel, W.; Aubke, F. Angew. Chem., Int. Ed. 2000, 39, 165.
doi: 10.6023/cjoc201507013 |
|
(b) Sayah, R.; Glegola, K.; Framery, E.; Dufaud, V. Adv. Synth. Catal. 2007, 349, 373.
doi: 10.6023/cjoc201507013 |
|
|
(c) Gholinejad, M.; Hamed F.; Biji, P. Dalton Trans. 2015, 44, 14293.
doi: 10.6023/cjoc201507013 |
|
|
(d) Zhang, Q.; Li, J.-H.; Zhao, X. Chin. J. Org. Chem. 2016, 36, 130 (in Chinese).
doi: 10.6023/cjoc201507013 |
|
|
(张强, 李继航赵鑫, 有机化学, 2016, 36, 130.)
doi: 10.6023/cjoc201507013 |
|
|
(e) Yang, P.-B.; Ma, R.; Bian, F.-L. ChemCatChem 2016, 8, 3746.
doi: 10.6023/cjoc201507013 |
|
|
(f) Sharma, S.; Nazir, R.; Pande, S.; Sarkar, B. R. ChemistrySelect 2017, 2, 8745.
doi: 10.6023/cjoc201507013 |
|
|
(g) Kunfi, A.; May, Z.; Németh, P.; London, G. J. Catal. 2018, 361, 84.
doi: 10.6023/cjoc201507013 |
|
|
(h) Liu, X.-M.; Tang, B.; Long, J.-L.; Zhang, W.; Liu, X. H.; Mirza, Z. Sci. Bull. 2018, 63, 502.
doi: 10.6023/cjoc201507013 |
|
|
(i) Fu, Y.-F.; Zou, Z.-J.; Tang, C.; Song, K.-P. Chin. J. Org. Chem. 2018, 38, 3106 (in Chinese).
doi: 10.6023/cjoc201507013 |
|
|
(付玉芳, 邹志娟, 唐成, 宋昆鹏, 有机化学, 2018, 38, 3106.)
doi: 10.6023/cjoc201507013 |
|
|
(j) Narkhede, N.; Uttam, B.; Rao, C. P. ACS Omega 2019, 4, 4908.
doi: 10.6023/cjoc201507013 |
|
| [21] |
(a) Wang, W.; Yang, Q.; Zhou, R.; Fu, H.-Y.; Zheng, X.-L.; Chen, H.; Li, R.-X. J. Organomet. Chem. 2012, 697, 1.
doi: 10.6023/cjoc201807040 |
|
(b) Wang, W.; Zhou, R.; Jiang, Z.-J.; Wang, K.; Fu, H.-Y.; Zheng, X.-L.; Chen, H.; Li, R.-X. Adv. Synth. Catal. 2014, 356, 616.
doi: 10.6023/cjoc201807040 |
|
|
(c) Wang, W.; Zhou, R.; Jiang, Z.-J.; Wang, X.; Fu, H.-Y.; Zheng, X.-L.; Chen, H.; Li, R.-X. Eur. J. Org. Chem. 2015, 2579.
doi: 10.6023/cjoc201807040 |
|
|
(d) Huang, J.; Fu, R.-H.; Jing, L.-H.; Qin, D.-B.; Huang, K.; Wang, W. Chin. J. Org. Chem. 2019, 39, 456 (in Chinese).
doi: 10.6023/cjoc201807040 |
|
|
(黄锦, 付荣辉, 敬林海, 秦大斌, 黄昆, 汪伟, 有机化学, 2019, 39, 456.)
doi: 10.6023/cjoc201807040 |
|
|
(e) He, H.-Y.; Wang, W.; Yu, X.-J.; Huang, J.; Jian, L.; Fu, H.-Y.; Zheng, X.-L.; Chen, H.; Li, R.-X. Eur. J. Org. Chem. 2016, 56169.
doi: 10.6023/cjoc201807040 |
|
| [22] |
Arumugam V.; Kaminsky W.; Bhuvanesh N. S. P.; Nallasamy D. RSC Adv. 2015, 5 59428.
doi: 10.1039/C5RA10973F |
| [23] | Lysén M.; Köhler K. Synthesis 2006, 4 692. |
| [24] |
Jansa J.; Jambor R. Appl. Organomet. Chem. 2016, 30 1036.
doi: 10.1002/aoc.3539 |
| [25] |
Ahmed J.; Chakraborty S.; Jose A.; Mandal S. K. J. Am. Chem. Soc. 2018, 140 8330.
doi: 10.1021/jacs.8b04786 |
| [26] |
Klein M.; Voigtmann U.; Haack T.; Erdinger L.; Boche G. Mutat. Res. 2000, 467 55.
doi: 10.1016/S1383-5718(00)00012-7 |
| [27] | Macé Y.; Raymondeau B.; Pradet C.; Blazejewski J. C.; Magnier E. Eur. J. Org. Chem. 2009 1390. |
| [28] |
Ernst J. B.; Rakers L.; Glorius F. Synthesis 2017, 49 260.
doi: 10.1055/s-0036-1588609 |
| [29] |
Duan X.-Y.; Li P.-B.; Zhu G.-R.; Fu C.-L.; Chen Q.; Huang X.; Ma S.-M. Org. Chem. Front. 2018, 5 3319.
doi: 10.1039/C8QO00781K |
| [30] |
Chiu C. C.; Chiu H. T.; Lee D. S.; Lu T. J. RSC Adv. 2018, 8 26407.
doi: 10.1039/C8RA04094J |
| [1] | Jing Wang, Linlin Wu, Qian Wang. Synthesis and Characterization of New Indeno[1,2-b]fluorene-6,12-dione Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 223-228. |
| [2] | Xuefeng Jia, Xiangjuan Tong. Recent Progress on Chan-Lam Coupling Reactions Catalyzed by Copper(II) Complexes [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2640-2658. |
| [3] | Li Pengshuai, Wu Yun, Bai Chaolumen, Bao Yongsheng. Insight into Catalytic Properties of Supported Palladium Nanoparticles Catalyzed ortho-Directed Sulfonylation [J]. Chinese Journal of Organic Chemistry, 2020, 40(7): 1991-1998. |
| [4] | Ouyang Yao, Xu Xiuhua, Qing Fengling. Oxidative Coupling Reactions of Arylboronic Acids and Fluoroform-Derived AgCF3 [J]. Chinese Journal of Organic Chemistry, 2020, 40(10): 3426-3430. |
| [5] | Huang Jin, Fu Ronghui, Jing Linhai, Qin Dabin, Huang Kun, Wang Wei. A Convenient Access to 3-Substituted Benzofuran Derivatives via Palladium Nanoparticles-Catalyzed Intramolecular Heck Reaction [J]. Chin. J. Org. Chem., 2019, 39(2): 456-462. |
| [6] | Dong Kun, Li Qiuyun, An Kang, Ma Junyi, Bai Zhongsheng, Liu Qiancai. Palladium-Catalyzed Syntheses of C3-Symmetric 9-Aryl Acridine Derivatives [J]. Chin. J. Org. Chem., 2018, 38(2): 416-424. |
| [7] | Liu Boqu, Yan Zhongfei, Quan Zhengjun. Palladium/Copper(I) Acetate-Promoted Desulfurative Coupling of Pyrimidine Thioether with Alkynes or Arylboronic Acids [J]. Chin. J. Org. Chem., 2018, 38(11): 3032-3038. |
| [8] | Jiang Haifang, Zhang Min, Zhang Li, Chen Yali, Zhu Ning, Song Liping, Deng Hongmei. Synthesis of 3,4-Diaryl-5-aryloxymethyl Isoxazole Derivatives [J]. Chin. J. Org. Chem., 2017, 37(9): 2399-2408. |
| [9] | Yan Xiaohui, Li Jiarong, Zhang Qi, Shi Daxin. Synthesis of Nitroarenes under Microwave [J]. Chin. J. Org. Chem., 2017, 37(6): 1450-1455. |
| [10] | Yuan Hang, Chen Huilian, Luo Zhibin, Gao Yuhua, Lu Hongfei. Synthesis of a Pd-Pyridine N-Heterocyclic Carbene Complex (NHC)-PdCl2(Py) and Its Efficient Application in Coupling Reaction [J]. Chin. J. Org. Chem., 2017, 37(11): 2948-2955. |
| [11] | Feng Cuilan, Hei Liying, Li Zhen, Liu Lantao. Magnetic Microporous Organic Polymer of 1,1'-Bi-2-naphtholSupported Palladium for Suzuki Coupling Reactions [J]. Chin. J. Org. Chem., 2016, 36(1): 179-184. |
| [12] | Hei Liying, Feng Cuilan, Li Zhen, Liu Lantao, Gui Jianzhou. Microporous Network Polyaniline Coated Magnetic Fe3O4 Nanoparticals Supported Palladium Catalyzed Suzuki and Heck Coupling Reactions [J]. Chin. J. Org. Chem., 2015, 35(8): 1673-1681. |
| [13] | Tang Yan, Yang Feifei, Nie Shipeng, Wang Lin Luo Zhibin, Lu Hongfei. Synthesis of a Carbene Complex and Its Application in Suzuki Reaction [J]. Chin. J. Org. Chem., 2015, 35(3): 705-711. |
| [14] | Lin Zhiqiang, Zeng Xiangchao, Meng Yuxia. Syntheses and Spectral Properties of N-Alkyl-5-arylindole-3-carboxaldehydes [J]. Chin. J. Org. Chem., 2015, 35(2): 490-496. |
| [15] | Xu Qing, Jia Xiaojuan, Li Xiaohui, Sun Qing, Zhou Yongbo, Yin Shuangfeng, Han Libiao. Copper-Catalyzed Aerobic Oxidative C-P Coupling of Arylboronic Acids and Diethyl Phosphite under Air [J]. Chin. J. Org. Chem., 2014, 34(7): 1340-1346. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||