Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (6): 2393-2400.DOI: 10.6023/cjoc202101058 Previous Articles Next Articles
ARTICLES
张涛a, 李念先a, 周楠茜b, 马雯a, 卫海沅a, 张冰欣a, 陈亮辉a, 海广范a, 段迎超a, 白素平a,*( )
)
收稿日期:2021-01-31
修回日期:2021-02-26
发布日期:2021-03-04
通讯作者:
白素平
基金资助:
Tao Zhanga, Nianxian Lia, Nanqian Zhoub, Wen Maa, Haiyuan Weia, Bingxin Zhanga, Lianghui Chena, Guangfan Haia, Yingchao Duana, Suping Baia( )
)
Received:2021-01-31
Revised:2021-02-26
Published:2021-03-04
Contact:
Suping Bai
Supported by:Share
Tao Zhang, Nianxian Li, Nanqian Zhou, Wen Ma, Haiyuan Wei, Bingxin Zhang, Lianghui Chen, Guangfan Hai, Yingchao Duan, Suping Bai. Design, Synthesis and Biological Evaluation of Novel Thiazole-Fused Glaucocalyxin A Derivatives[J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2393-2400.
| Entry | Compd. | Reaction conditionsa | Yield/% |
|---|---|---|---|
| 1 | 1 | PyHBr3 (1.2 equiv.), THF, 0 ℃, 1 h | 51 |
| 2 | 1 | PyHBr3 (1.2 equiv.), THF, –20 ℃, 12 h | 36 |
| 3 | 1 | PyHBr3 (1.2 equiv.), THF, 20 ℃, 1 h | 41 |
| 4 | 1 | PyHBr3(1.5 equiv.), THF, 0 ℃, 1 h | 30 |
| 5 | 1 | PyHBr3 (1.1 equiv.), THF, 0 ℃, 0.5 h | 56 |
| 6 | 1 | NBS (1.2 equiv.), THF, 0 ℃, 12 h | <10 |
| 7 | 3 | PyHBr3 (1.1 equiv.), THF, 0 ℃, 12 h | 40 |
| 8 | 3 | NBS (1.2 equiv.), THF, 0 ℃, Et3N, 12 h | <10 |
| 9 | 3 | PyHBr3 (1.0 equiv.), THF, 20 ℃, 6 h | 32 |
| Entry | Compd. | Reaction conditionsa | Yield/% |
|---|---|---|---|
| 1 | 1 | PyHBr3 (1.2 equiv.), THF, 0 ℃, 1 h | 51 |
| 2 | 1 | PyHBr3 (1.2 equiv.), THF, –20 ℃, 12 h | 36 |
| 3 | 1 | PyHBr3 (1.2 equiv.), THF, 20 ℃, 1 h | 41 |
| 4 | 1 | PyHBr3(1.5 equiv.), THF, 0 ℃, 1 h | 30 |
| 5 | 1 | PyHBr3 (1.1 equiv.), THF, 0 ℃, 0.5 h | 56 |
| 6 | 1 | NBS (1.2 equiv.), THF, 0 ℃, 12 h | <10 |
| 7 | 3 | PyHBr3 (1.1 equiv.), THF, 0 ℃, 12 h | 40 |
| 8 | 3 | NBS (1.2 equiv.), THF, 0 ℃, Et3N, 12 h | <10 |
| 9 | 3 | PyHBr3 (1.0 equiv.), THF, 20 ℃, 6 h | 32 |
| Compd. | IC50a (µmol•L–1) | |||||
|---|---|---|---|---|---|---|
| Hep G2 | NCI-H460 | JEG-3 | K562 | HL-60 | HeLa | |
| 5 | 3.97±0.09 | 5.27±0.21 | 5.19±0.06 | 10.12±0.20 | 2.40±0.15 | 2.33±0.11 |
| 6 | 3.08±0.06 | 4.07±0.15 | 1.13±0.14 | 5.63±0.48 | 0.82±0.03 | 0.56±0.02 |
| 7 | 3.20±0.30 | 3.31±0.24 | 2.13±0.37 | 4.00±0.21 | 0.84±0.06 | 2.17±0.06 |
| 8 | 1.81±0.01 | 2.47±0.25 | 3.04±0.09 | 3.25±0.09 | 0.90±0.06 | 0.51±0.05 |
| 9 | 2.53±0.13 | 3.35±0.18 | 3.70±0.12 | 4.08±0.29 | 1.19±0.04 | 2.19±0.04 |
| 10 | 4.14±0.37 | 3.95±0.16 | 6.03±0.67 | 7.33±0.72 | 1.50±0.10 | 2.02±0.08 |
| 11 | 6.66±0.53 | 8.07±0.34 | 18.81±1.92 | 6.54±0.41 | 3.31±0.15 | 4.47±0.03 |
| 12 | 3.15±0.08 | 3.18±0.26 | 4.97±0.46 | 6.03±0.30 | 2.02±0.09 | 2.12±0.11 |
| 13 | 10.70±0.29 | 9.69±0.40 | 9.71±0.21 | 9.72±0.54 | 3.09±0.19 | 5.28±0.03 |
| 14 | 5.17±0.34 | 4.83±0.04 | 6.28±0.10 | 22.49±0.75 | 2.42±0.11 | 2.49±0.05 |
| GLA | 3.94±0.07 | 8.99±0.18 | 4.25±0.08 | 7.20±0.17 | 6.46±0.05 | 8.19±1.96 |
| Adriamycin | 0.50±0.01 | 1.73±0.02 | 0.52±0.02 | 5.15±0.11 | 1.73±0.02 | 0.69±0.01 |
| Compd. | IC50a (µmol•L–1) | |||||
|---|---|---|---|---|---|---|
| Hep G2 | NCI-H460 | JEG-3 | K562 | HL-60 | HeLa | |
| 5 | 3.97±0.09 | 5.27±0.21 | 5.19±0.06 | 10.12±0.20 | 2.40±0.15 | 2.33±0.11 |
| 6 | 3.08±0.06 | 4.07±0.15 | 1.13±0.14 | 5.63±0.48 | 0.82±0.03 | 0.56±0.02 |
| 7 | 3.20±0.30 | 3.31±0.24 | 2.13±0.37 | 4.00±0.21 | 0.84±0.06 | 2.17±0.06 |
| 8 | 1.81±0.01 | 2.47±0.25 | 3.04±0.09 | 3.25±0.09 | 0.90±0.06 | 0.51±0.05 |
| 9 | 2.53±0.13 | 3.35±0.18 | 3.70±0.12 | 4.08±0.29 | 1.19±0.04 | 2.19±0.04 |
| 10 | 4.14±0.37 | 3.95±0.16 | 6.03±0.67 | 7.33±0.72 | 1.50±0.10 | 2.02±0.08 |
| 11 | 6.66±0.53 | 8.07±0.34 | 18.81±1.92 | 6.54±0.41 | 3.31±0.15 | 4.47±0.03 |
| 12 | 3.15±0.08 | 3.18±0.26 | 4.97±0.46 | 6.03±0.30 | 2.02±0.09 | 2.12±0.11 |
| 13 | 10.70±0.29 | 9.69±0.40 | 9.71±0.21 | 9.72±0.54 | 3.09±0.19 | 5.28±0.03 |
| 14 | 5.17±0.34 | 4.83±0.04 | 6.28±0.10 | 22.49±0.75 | 2.42±0.11 | 2.49±0.05 |
| GLA | 3.94±0.07 | 8.99±0.18 | 4.25±0.08 | 7.20±0.17 | 6.46±0.05 | 8.19±1.96 |
| Adriamycin | 0.50±0.01 | 1.73±0.02 | 0.52±0.02 | 5.15±0.11 | 1.73±0.02 | 0.69±0.01 |
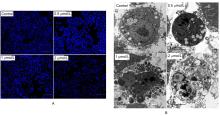



| [1] |
Sharifi-Rad, J.; Ozleyen, A.; Boyunegmez Tumer, T.; Oluwaseun Adetunji, C.; El Omari, N.; Balahbib, A.; Taheri, Y.; Bouyahya, A.; Martorell, M.; Martins, N.. C. Cho, W. Biomolecules 2019, 9,679.
doi: 10.3390/biom9110679 |
| [2] |
Wong, A. S. T.; Che, C. M.; Leung, K. W. Nat. Prod. Rep. 2015, 32,256.
doi: 10.1039/C4NP00080C |
| [3] |
Li, G.; Lou, H. X.. Med. Res. Rev. 2018, 38,1255.
|
| [4] |
Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Nat. Chem. 2016, 8,531.
doi: 10.1038/nchem.2479 pmid: 27219696 |
| [5] |
Newman, D. J.; Cragg, G. M. J. Nat. Prod. 2016, 79,629.
doi: 10.1021/acs.jnatprod.5b01055 pmid: 26852623 |
| [6] |
Zhang, W.; Huang, Q.; Hua, Z. C. Front. Biol. 2010, 5,540.
doi: 10.1007/s11515-010-0610-8 |
| [7] |
Li, D.; Han, T.; Liao, J.; Hu, X.; Xu, S.; Tian, K.; Gu, X.; Cheng, K.; Li, Z.; Hua, H.; Xu, J. Int. J. Mol. Sci. 2016,17.
|
| [8] |
Wang, M.; Li, H.; Xu, F.; Gao, X.; Li, J.; Xu, S.; Zhang, D.; Wu, X.; Xu, J.; Hua, H.; Li, D. Eur. J. Med. Chem. 2018, 156,885.
doi: 10.1016/j.ejmech.2018.07.052 |
| [9] |
Xiang, Z.; Wu, X.; Liu, X.; Jin, Y. Nat. Prod. Res. 2014, 28,2221.
doi: 10.1080/14786419.2014.934235 |
| [10] |
Wang, F. D.; Wang, T.; Wu, A. A.; Ding, L.; Wang, H. Q. Chin. J. Chem. Phys. 2009, 22,275.
doi: 10.1088/1674-0068/22/03/275-284 |
| [11] |
Gao, L. W.; Zhang, J.; Yang, W. H.; Wang, B.; Wang, J. W. Toxicol in Vitro 2011, 25,51.
doi: 10.1016/j.tiv.2010.09.006 |
| [12] |
Guo, J.; Li, B.; Ma, W.; Pitchakuntla, M.; Jia, Y. Angew. Chem. Int. Ed. 2020, 59,15195.
doi: 10.1002/anie.v59.35 |
| [13] |
Cheng, D. D.; Zhang, Y. X.; Wang, J. M.; Bai, S. P. Chin. J. Org. Chem. 2019, 39,1794.
doi: 10.6023/cjoc201903013 |
| [14] |
Liu, H. C.; Qiao, L. M.; Zheng, W.; Xiang, Z. B.; Chen, H. S.; Yu, S. C.; Zhang, D. Z.; Wang, T.; Zhang, Y. F.; Jin, Y. S. Anti-Cancer Agents Med. Chem. 2020, 20,1241.
doi: 10.2174/1871520620666200302114550 |
| [15] |
Yang, J.; Liu, Y.; Xue, C.; Yu, W.; Zhang, J.; Qiao, C. Eur. J. Med. Chem. 2014, 86,235.
doi: 10.1016/j.ejmech.2014.08.061 |
| [16] |
Yuan, Y. L.; Ye, D. D.; Liang, H. J.; Zhou, N. Q.; Hai, G. F.; Zhang, Y. X.; Bai, S. P. Planta Med. 2012, 78,589.
doi: 10.1055/s-0031-1298265 |
| [17] |
Ren, L.; Wang, J.; Chen, G. Drug Delivery 2019, 26,309.
doi: 10.1080/10717544.2019.1568623 |
| [18] |
Ding, C.; Zhang, Y.; Chen, H.; Yang, Z.; Wild, C.; Chu, L.; Liu, H.; Shen, Q.; Zhou, J. J. Med. Chem. 2013, 56,5048.
doi: 10.1021/jm400367n |
| [19] |
Dahiya, R.; Dahiya, S.; Fuloria, N. K.; Kumar, S.; Mourya, R.; Chennupati, S. V.; Jankie, S.; Gautam, H.; Singh, S.; Karan, S. K.; Maharaj, S.; Fuloria, S.; Shrivastava, J.; Agarwal, A.; Singh, S.; Kishor, A.; Jadon, G.; Sharma, A. Mar. Drugs 2020, 18,329.
doi: 10.3390/md18060329 |
| [20] |
Sharma, P. C.; Bansal, K. K.; Sharma, A.; Sharma, D.; Deep, A. Eur. J. Med. Chem. 2020, 188,112016.
doi: 10.1016/j.ejmech.2019.112016 |
| [21] |
Ayati, A.; Emami, S.; Moghimi, S.; Foroumadi, A. Future Med. Chem. 2019, 11,1929.
doi: 10.4155/fmc-2018-0416 |
| [22] |
Lin, Z.; Wang, Z.; Zhou, X.; Zhang, M.; Gao, D.; Zhang, L.; Wang, P.; Chen, Y.; Lin, Y.; Zhao, B.; Miao, J.; Kong, F. Cell Death Dis. 2020, 11,551.
doi: 10.1038/s41419-020-02746-w |
| [23] |
Qiao, Q.; So, S. S.; Goodnow, R. A. Org. Lett. 2001, 3,3655.
pmid: 11700105 |
| [24] |
Yoshio, T.; Tetsuro, F.; Akira, U. Chem. Lett. 1981, 10,1229.
doi: 10.1246/cl.1981.1229 |
| [25] |
Liang, H. J.; Zhang, Y. X.; Hai, G. F.; Bai, S. P.; Yuan, Y. L.; Ye, D. D.; Zhou, N. Q. Planta Med. 2012, 78,589.
doi: 10.1055/s-0031-1298265 |
| [26] |
Liu, W.; Fan, B.-L.; Guo, H. Y.; Yuan, Y. L.; Liang, H.-J.; Bai, S. P.. Nat. Prod. Res. 2013, 27,1388.
doi: 10.1080/14786419.2012.746339 |
| [1] | Xiaoying Jia, Jiaxia Pu, Lirong Han, Qinghan Li. Research Progress in the Synthesis of Benzo[d]pentamembered Heterocyclic Thioethers Containing Two Heteroatoms [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 18-40. |
| [2] | Bozhen Wang, Jie Zhang, Chunhui Nian, Mingming Jin, Miaomiao Kong, Wulan Li, Wenfei He, Jianzhang Wu. Synthesis and Antitumor Activity of 3,4-Dichlorophenyl Amides [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 232-241. |
| [3] | Panxing Pang, Rong Ning, Chuang Zhu, Wenjie Huang, Xianli Ma, Caina Jiang, Fangyao Li, Xiaoqun Zhou. Synthesis and in Vitro Antitumor Activity of Matrine Semicarbazide Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2126-2135. |
| [4] | Kanghui Duan, Junlong Tang, Wanqing Wu. Recent Advances in the Synthesis of Fused Heterocyclic Compounds and Their Antitumor Activities [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 826-854. |
| [5] | Meng Li, Dongguo Xia, Yunxiao Wang, Xiang Cheng, Jiexiu Gong, Yao Chen, Xianhai Lü. Design, Synthesis and Antifungal Bioactivity Evaluation of Thiazole Benzoate Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 686-696. |
| [6] | Chujie Liao, Hongyao Ruan, Junfeng Jiang, Lun Luo, Yanggen Hu. Synthesis and Activity Evaluation of 3-Aryl-2-imino-benzo[e][1,3]-oxazin-4-ol Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 763-770. |
| [7] | Meng Liu, Yanru Huang, Xiaofei Sun, Lijun Tang. An “Aggregation-Induced Emission+Excited-State Intramolecular Proton Transfer” Mechanisms-Based Benzothiazole Derived Fluorescent Probe and Its ClO– Recognition [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 345-351. |
| [8] | Weiqin Liu, Lihui Shao, Chengpeng Li, Yayu Zou, Haitao Long, Yan Li, Qiangsheng Ge, Zhenchao Wang, Guiping Ouyang. Synthesis and Antitumor Activity of 3-Hydrazone Quinazolinone Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 214-222. |
| [9] | Guangping Liang, Wei Wang, Xuxiu Zhu, Guangyan Liang, Jun Yang, Daoping Wang. Synthesis and in Vitro Anti-tumor Activity of Novel Spliced Compounds of Zidovudine and 4-Anilinoquinazolines [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2793-2805. |
| [10] | Dongyan Hu, Guangtian Han, Xi'an Li, Huazhong Ren, Lirong Yue, Li Guo, Jiafu Feng. Synthesis and Evaluation in vitro of Novel Harmine Derivatives as Anticancer Activity Agents [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1863-1871. |
| [11] | Lu Xue, Lihua Zhang, Chengyu Zhang, Xin Zhao, Weifan Dang, Zhaoxin Wang, Chunhua Wang, Tongchuan Suo, Xiaohui Yan. Discovery of Tiancimycin Congeners from Streptomyces sp. CB03234-S [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1241-1247. |
| [12] | Siyu Zhu, Xinyu Huo, Qin Ma, Wei Chen, Jie Zhang, Liang Guo. Design, Synthesis, and Antitumor Activity of β-Carboline-Benzimidazole Hybrids [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1129-1135. |
| [13] | Tao Zhang, Haiyuan Wei, Wen Ma, Zhangyuan Li, Panpan Hu, Nanqian Zhou, Jianchao He, Ting Li, Mingming Su, Suping Bai. Synthesis and Antiproliferative Activity Evaluation of Novel Glaucocalyxin A-1,2,3-Triazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3668-3683. |
| [14] | Shu Chen, Yingying Shao, Xinhao Fu, Qingwu Chen, Xiaohua Du, Chengxia Tan. Design, Synthesis and Insecticidal Activities of Pyridyl Thiazole Diamide Compounds [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3870-3879. |
| [15] | Honglin Dai, Xiaojie Si, Lingling Chi, Hao Wang, Chao Gao, Zhengjie Wang, Limin Liu, Jiajie Ma, Fuqiang Yu, Hongmin Liu, Yu Ke, Qiurong Zhang. Synthesis and Antitumor Activity Evaluation of 2,4,6-Trisubstituted Quinazoline Derivatives Containing Thiazole Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3853-3862. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||